Wednesday, January 2, 2013
Ion channel selectivity to ions with selectivity filters and other structures
Ion channels like proton (H+), sodium, potassium, calcium and chlorine channels let ions through cell membranes selectively by having narrow point in the tunnel that can only let through ions (or ion-water complexes) of certain size and charge. Charge selectivity depends on electrostatic attraction or repulsion depending on which amino acids are inside ion channel. Much of ion channel proteins is made of helical amino acid chains (alpha-helix) and these have tendency to align positive and negative charges consistently through helix (negative parts aimed at one side of cell membrane and positive parts to other parts).
Chart of amino acids. Pink areas are parts that connect amino acids together in proteins/peptides and other parts determine which charge amino acid has in proteins. "Polar basic" amino acids usually have positive charge while "polar acidic" amino acids have negative charge but collisions with reactive elements can have less predictable effect on charges.
Voltage sensitivity can be achieved with positively charged amino acids through membranes. Above illustration is about sodium channel but many ion channels have similar 4 repeating groups. Those 4 repeating groups form 1 pore through cell membrane that is about 0,3-0,5 nanometers wide (radius of sodium is about 0,18 nm). 4th helix in all of these groups detect membrane voltage by being attracted to more negative side of membrane and with its pull it closes or opens depending on direction of charges. During resting phase cell is more negative inside and during electric impulse inside becomes more positive than outside. Larger voltage difference pulls-pushes charged parts with greater mechanical force. Other voltage gated ion channels have likely similar mechanisms involved but in others both charges could be in use as voltage detectors.
Size selectivity can depend on size like in case of relatively tiny sodium channel letting small sodium ion through but not larger potassium or calcium. Potassium channels have different mechanisms as they don't let smaller sodium through. Ions like sodium and potassium both attract water molecules and this group of atoms can behave like larger particle. In potassium channel larger pore reaches water around ion and removes some of it but can't do it for water around smaller sodium. Suspected mechanisms include oxygens in C=O groups stabilizing potassium to stay in channel while sodium wouldn't be stabilized and is unlikely to pass narrow tunnel (potassium channels have about 1/10 000 chance of letting sodium through instead of potassium). As channel could fit few potassium atoms it is also possible that they repulse each others and push each other out of the channel giving it maximum ion flow of up to 100 million potassium ions per second (in neurons opening for about 1 millisecond). One additional possible factor is that helical proteins have charged areas in predictable areas. In loops of helices oxygens are on the side towards C terminus of protein and NH are towards N terminus. This creates repeating rows of H, N and O that could attract positive ions in that sequence as electronegativity determines which atoms gets what charge and after that electrostatic interactions start to work on ions. Voltage sensing in potassium channels is controlled by charged glutamine acids and lysines which attract to side of cell suitable for their charge and distort potassium channel in process. Common potassium channels on neurons open only if inside of cell becomes positively charged.
Sodium channel pore parts have mainly negatively charged amino acids and mutations that reduce negative charge there slow the flow of sodium through the channel. Replacing weakly positive lysine (this amino acid could behave like negative charge to calcium due to electronegativity of hydrogen and nitrogen making them pull electrons from calcium) or uncharged alanine with glutamine acid make sodium channel behave more like calcium channel.
Chlorine channel in E.coli has 3 binding sites for chlorine atoms. At least outermost of these sites can attract carboxylic group from glutamine acid and block the ion channel.
Calcium channel can let sodium through more easily if negatively charged glutamine acids in certain locations (one of 3 seems enough) gets replaced by uncharged glutamine or alanine. At least 3 glutamine acids line inside of calcium channels and seem to participate in selectively letting positive ions through.
Proton channels are so selective to H+ ions that in that study authors didn't detect other ions getting through it. When they replaced aspartic acid with similarly charged glutamic acid it stayed selective to H+ but when Asp was replaced with uncharged amino acid the channel stopped conducting ions or was letting selectively negative ions through.
Ion channels tend to be covered with sugar type molecules (glycosylated) and amount of glycosylation influences sensitivity to voltage.
Possible reason why channels need those could be because sugary substances like syrups and starch create viscous goo that slows down flow of fluid. Even mucus proteins seem to get their gooey consistency from glucose type additives on it's protein chain.
For proteins this slowdown means that charged particles don't fly by so fast and leave more time for their electric charges to pull-push other charges around.
Calculator for finding how fast is molecule or atom with chosen mass on chosen temperature. At body temperature sodium have average speed of 470 m/s (1700 km/h). Water is about 10% faster than sodium. Water freezes if its molecules are slower than 502 m/s at 0 C and boil at 587 m/s with electrostatic attractions in form of hydrogen bonds keeping ice or liquid water together at these speeds. Overall water and ions move about 1 m/s (~3 km/h) per each degree C. Calcium and potassium are have speeds of around 350 m/s or 1290 km/h. At such sometimes supersonic speeds ions don't leave each other much time to react to each others charges. In liquid water particles are still fast but they keep attracting each others and also collide often which slows average observable speed to what can be seen with color diffusing through water. By adding slimy consistency particles slow down even further and leave electric attractors or repulsors in proteins or artificial nano-devices more time to push-pull ions in predictable way.
Sunday, December 23, 2012
Bits about slime
In nature slime is mostly composed of fiber like proteins that are often covered by many glucose groups. Common type of mucus proteins in mammals is mucin and this is also present in fish slime. Mucins can have molecular mass of 1-10 million hydrogen atoms and in addition to being long fibers they also form many sulfur "bridges" between cysteine parts that connect random parts of fibers making it possible to connect several different mucin molecules with sulfur. Having glycose molecules attached makes mucin and other proteins likelier to attract water and avoid degradation by proteases.
One possible use for slime could be in making strong strands although as proteins they probably degrade if bacteria and humidity could touch it. Hagfishes produce lots of slime for self-defense (as shown in Hammonds Miracles of Nature 3 scene). After drying it turns into strong fiber close to the strength of spider silk although with less complex structure.
Prostate secretes some proteins that break up proteins making entire mixture more fluid. For example PSA (prostate-specific antigen) breaks up proteins that kept semen more solid and liquify it for ejaculating.
Slime produced by any body part is likely to smell "fishy" as they share exactly same odor molecule. One receptor that releases slime is acetylcholine and it eventually breaks down to trimethylamine which smells fishy and this could be felt in the mucus/slime of nose, mouth, vagina, penis (due to slime part of semen) and fishes.
One possible use for slime could be in making strong strands although as proteins they probably degrade if bacteria and humidity could touch it. Hagfishes produce lots of slime for self-defense (as shown in Hammonds Miracles of Nature 3 scene). After drying it turns into strong fiber close to the strength of spider silk although with less complex structure.
Prostate secretes some proteins that break up proteins making entire mixture more fluid. For example PSA (prostate-specific antigen) breaks up proteins that kept semen more solid and liquify it for ejaculating.
Slime produced by any body part is likely to smell "fishy" as they share exactly same odor molecule. One receptor that releases slime is acetylcholine and it eventually breaks down to trimethylamine which smells fishy and this could be felt in the mucus/slime of nose, mouth, vagina, penis (due to slime part of semen) and fishes.
Wednesday, November 7, 2012
Magnetism and electron configuration
In short atoms and molecules are repelled by magnets (diamagnetic) if they have no unpaired electrons (only electrons with 2 different spins in orbitals) but are somewhat attracted to magnets (paramagnetic) when they have at least 1 unpaired (electron with 1 spin in orbital) electron in their outermost electron layer. Diamagnetism is weakest form of magnetism and due to this weakness it shows up usually when material has no unpaired electron. Also core electrons deeper in atom contribute to diamagnetism due to aligning with outside magnetism and causing internal magnetic field that pushes away from outside magnetic field. Diamagnetic response is believed to have similar mechanism to larger scale magnetic responses as outside magnetic field that can move electrons in material cause magnetic field in that material (superconductor, ring of wire, benzene ring or just atom) that tries to repel outside magnetic field.
Some of the more stronger room temperature diamagnets are purely carbon compounds like graphene, pyrolytic carbon and diamonds. Carbon has connection to 4 other atoms and in these materials all 4 are paired making all carbon atom electrons diamagnetic.
Water is weakly diamagnetic (superconductor are about 100 000 times more diamagnetic than water) and its stream can be pushed few millimeters away from strong magnet (clip). Another more sensitive way to test it is to put water container on floating vessel and slowly push it by holding magnet near water (clip). As animals are mostly water they can be floated above strong enough magnets. Frogs may float at around 10 tesla (clip). Humans have been tested in at least 7 tesla MRIs and while they didn't float they had some additional side effects like flashes after changing direction of magnetic field. These flashes were suspected to be caused by diamagnetic responses in parts of retinal rod cells that realign themselves and by pressing against each other they may activate each others. Rhodopsin is the light sensitive protein in rod cells and at least these proteins are also diamagnetic.
Copper is one of the few diamagnetic metals (clip) and copper pipes can slow down strong magnets falling through them.
Main difference between ferromagnets and paramagnets is that ferromagnets don't need outside magnetic field to become and stay magnetic. Both of these arise from unpaired electron but crystal structure of ferromagnets keeps them from losing magnetism due to heat movements that remove magnetism from paramagnetic materials with random movements. Permanent magnets use crystal structure that are magnetic in certain directions while resisting alignments in other directions. In production of permanent magnets all these crystals get aligned in one direction so entire material would spontaneously magnetize in same direction with enough stability to stay that way below curie temperatures.
Table showing unpaired electrons for single ions. Single hydrogen atom is purely paramagnetic and attracted to magnets but it becomes diamagnetic after becoming hydrogen molecule with all electrons paired. Transition metals have unfilled d orbitals that make them paramagnetic or ferromagnetic. Electron are in increasingly more diamagnetic configurations in lower rows of transition metals and most famous strong magnets use elements from 1st row of transition metals.
Electron pairing is usually shown with single or 2 opposite oriented arrows. Transition metals usually have electron orbitals with same energies (degenerate orbitals) and electrons can move freely as gas in transition metals between orbitals. Magnetism can arise in combination with other element. According to crystal field theory electrons stop having equal energy if other element has electrostatic effect on metal atom by pulling or pushing electrons to 1 side of atom.
2 rows show orbitals on side of metal closer to and further from other element that had electrostatic effect. Electrons closer to neighboring atom gets some extra energy from electrostatic interaction and if energy difference between orbitals is small then electrons can occupy them singly with high spin electrons (arrow up).
If resulting interaction between atoms makes some orbitals harder to access so it would take less energy to combine with other electron on same orbital then electrons tend to pair up (low-spin) and not go on new orbital unless all single electrons are paired.
Some of the more stronger room temperature diamagnets are purely carbon compounds like graphene, pyrolytic carbon and diamonds. Carbon has connection to 4 other atoms and in these materials all 4 are paired making all carbon atom electrons diamagnetic.
Water is weakly diamagnetic (superconductor are about 100 000 times more diamagnetic than water) and its stream can be pushed few millimeters away from strong magnet (clip). Another more sensitive way to test it is to put water container on floating vessel and slowly push it by holding magnet near water (clip). As animals are mostly water they can be floated above strong enough magnets. Frogs may float at around 10 tesla (clip). Humans have been tested in at least 7 tesla MRIs and while they didn't float they had some additional side effects like flashes after changing direction of magnetic field. These flashes were suspected to be caused by diamagnetic responses in parts of retinal rod cells that realign themselves and by pressing against each other they may activate each others. Rhodopsin is the light sensitive protein in rod cells and at least these proteins are also diamagnetic.
Copper is one of the few diamagnetic metals (clip) and copper pipes can slow down strong magnets falling through them.
Main difference between ferromagnets and paramagnets is that ferromagnets don't need outside magnetic field to become and stay magnetic. Both of these arise from unpaired electron but crystal structure of ferromagnets keeps them from losing magnetism due to heat movements that remove magnetism from paramagnetic materials with random movements. Permanent magnets use crystal structure that are magnetic in certain directions while resisting alignments in other directions. In production of permanent magnets all these crystals get aligned in one direction so entire material would spontaneously magnetize in same direction with enough stability to stay that way below curie temperatures.
Table showing unpaired electrons for single ions. Single hydrogen atom is purely paramagnetic and attracted to magnets but it becomes diamagnetic after becoming hydrogen molecule with all electrons paired. Transition metals have unfilled d orbitals that make them paramagnetic or ferromagnetic. Electron are in increasingly more diamagnetic configurations in lower rows of transition metals and most famous strong magnets use elements from 1st row of transition metals.
Electron pairing is usually shown with single or 2 opposite oriented arrows. Transition metals usually have electron orbitals with same energies (degenerate orbitals) and electrons can move freely as gas in transition metals between orbitals. Magnetism can arise in combination with other element. According to crystal field theory electrons stop having equal energy if other element has electrostatic effect on metal atom by pulling or pushing electrons to 1 side of atom.
2 rows show orbitals on side of metal closer to and further from other element that had electrostatic effect. Electrons closer to neighboring atom gets some extra energy from electrostatic interaction and if energy difference between orbitals is small then electrons can occupy them singly with high spin electrons (arrow up).
If resulting interaction between atoms makes some orbitals harder to access so it would take less energy to combine with other electron on same orbital then electrons tend to pair up (low-spin) and not go on new orbital unless all single electrons are paired.
Sunday, October 28, 2012
Electronegativity and reactivity of molecules
In general molecules are more stable if there is large difference between electronegativities of their atoms but less stable and more energetically reacting if they are made of atoms with similar electronegativites (EN) excluding those elements with 4 free electrons like carbon that can form strong cubical crystal structure.
For example most metals react with oxygen when they could come in contact with that. Also pure sodium and other alkali metals start burning in water but they lose reactivity after combining with chlorine or other element from opposite side of periodic table. While nitrogen doesn't react easily with organic materials it can react in contact with lithium or magnesium. EN difference of 1,5 or more is usually enough to start energetic (often flaming) chemical reaction without any need to add heat but with smaller differences heat or catalyst are needed like between carbon and oxygen.
Energy releasing metabolism and burning usually create molecules with larger difference of EN between its atoms than before. For example fats and sugars often have mainly carbon and hydrogen connected to each other which have 0,35 difference in EN but after turning them into CO2 (0,89) and water (1,2 difference) this difference grows and resulting molecules need much energy (heat or energetic electric current) to break those up.
Precious metals in middle columns are relatively nonreactive or resistant to most acids, oxygen and other reactive substances compared to other metals. Among the most stable metals are metals with EN at least 2,2 (or more) like gold and those with less EN can rust like silver but others between like Mo usually need heat to start combining with oxygen without visibly rusting in room temperature air.
Many infrared sensors use unstable molecules that create electron flow with even less energy than visible light provides. These are often cooled to 60-100 degrees above absolute zero to avoid detectors from blinding itself by creating electron currents due to room temperature and cooling is commonly needed to see room temperature heat. At the same time these same sensors without cooling are usually only useful for detecting temperatures that are hundreds of degrees above room temperature. As example PbSe has elements with EN differences of 0,2 and can detect infrared with 5-6 micrometer wavelength. Heat from human body is about 10 micrometer infrared radiation.
One of the most sensitive infrared sensor materials is mixture of Hg, Te and Cd which can detect almost 2-3 times less energetic infrared radiation than PbSe and it has about 2 times smaller difference in EN. Due to sensitivity HgTeCd has to be about 77 degrees above absolute zero to detect weaker infrared wavelength (up to ~12 micrometer wavelength for this mixture).
Thursday, October 18, 2012
Few comments about thiamine (vitamin B1) deficiency
Awareness about thiamine/vitamin B1 (other vitamin B types can run out together with B1) is maybe most important to those who use drugs that influence GABA and acetylcholine levels. GABA influencing drugs include ethanol, GHB and almost all sedatives like benzodiazepines and barbiturates. Acetylcholine is needed to move, remember stuff and to keep many glands working so most drugs that cause dryness in mouth, skin, mucus membranes and eyes probably block acetylcholine activity.
Thiamine is needed for at least 3 enzymes that produce energy from carbohydrates. It usually becomes effective for proteins after it gets 2 phosphate groups added to it. Alcohol stops thiamine absorption from stomach and also slows its activation by slower addition of phosphate groups (that last reaction is slowed because it needs magnesium and ethanol also lowers magnesium levels). While all cells seem to need it, heart and neurons are most sensitive. Serious lack of B1 can cause coma leading to death or deadly heart failure. It is also needed to produce GABA, acetylcholine and building materials to proteins, myelin and DNA plus many other substances. Usually thiamine levels become low if there are problems with absorbing it from intestines due to alcohol and drugs or due to health problems like chronic diarrhea, vomiting, stomach surgery or excessive urination with diuretics. Thiamine requirements are about 0,33 mg for each 1000 kcal of nutrients eaten. Meat is one main source of thiamine like also whole grains and brown rice. White rice on other hand cause lack of thiamine which is often called beriberi. Wernicke-Korsakoff syndrome is common name for this deficiency if caused by chronic alcoholism.
Common symptoms of thiamine deficiency: weakness, apathy, faster heart rate, weaker reflexes, unwanted eye movements, trouble breathing, possibly fluid in lungs related to heart problems, edema of lower legs and droopy eyelids. In case of Korsakoff syndrome (more extreme deficiency) it can cause problems with remembering past, learning new memories and may lead to memories of events that didn't happen (confabulation). Alcoholic delirium and brain damage are likely to come from thiamine deficiency. These last memory problems are somewhat common if brain is very low on acetylcholine.
Un-cited personal part: i recently noticed that almost all the unwanted side effects my sedative (pregabalin) causes overlapped with thiamine deficiency. I've experienced almost all previously mentioned temporary symptoms with that except serious memory problems. Pregabalin blocks calcium channels and it has mostly weak effect on all neurotransmitters (each is released after calcium triggers release). During minor withdrawal phase ~12 after dosing i often notice weird weakness and changes in posture with very foggy thinking with runny nose. Maybe it was because GABA and acetylcholine were being released too fast after calcium channel normalized and ran low so thiamine was diverted to producing those neurotransmitters and after awhile also run low causing deficiency symptoms. While inside cells neurotransmitters should preserve well but after releasing they get broken up often in split second. For example acetylcholine activates muscles controlled by willpower and this effect on muscles disappears fast because it gets broken up fast outside cells by acetylcholinesterase (each enzyme molecule breaking about 25 000 acetylcholine molecules per second). Acetylcholine is involved in nose mucus gland activation so anything causing that could cause problems with thiamine reserves. After noticing that it could explain why these outwardly visible symptoms happened i almost quit pregabalin overnight after taking it ~300 mg daily for over 3 years and got rid of these symptoms (surprising lack of withdrawals after 24 hour pause). I suspect most antipsychotics and sedatives could cause this effect on small scale but ethanol is still the most obvious cause for thiamine deficiency with worst magnitude that i know about. One nonacademic source listed diuretics, nicotine (binding with nicotinic acetylcholine receptors) and barbiturates (GABA receptor blockers) as substances that can adversely affect thiamine levels.
Thiamine is needed for at least 3 enzymes that produce energy from carbohydrates. It usually becomes effective for proteins after it gets 2 phosphate groups added to it. Alcohol stops thiamine absorption from stomach and also slows its activation by slower addition of phosphate groups (that last reaction is slowed because it needs magnesium and ethanol also lowers magnesium levels). While all cells seem to need it, heart and neurons are most sensitive. Serious lack of B1 can cause coma leading to death or deadly heart failure. It is also needed to produce GABA, acetylcholine and building materials to proteins, myelin and DNA plus many other substances. Usually thiamine levels become low if there are problems with absorbing it from intestines due to alcohol and drugs or due to health problems like chronic diarrhea, vomiting, stomach surgery or excessive urination with diuretics. Thiamine requirements are about 0,33 mg for each 1000 kcal of nutrients eaten. Meat is one main source of thiamine like also whole grains and brown rice. White rice on other hand cause lack of thiamine which is often called beriberi. Wernicke-Korsakoff syndrome is common name for this deficiency if caused by chronic alcoholism.
Common symptoms of thiamine deficiency: weakness, apathy, faster heart rate, weaker reflexes, unwanted eye movements, trouble breathing, possibly fluid in lungs related to heart problems, edema of lower legs and droopy eyelids. In case of Korsakoff syndrome (more extreme deficiency) it can cause problems with remembering past, learning new memories and may lead to memories of events that didn't happen (confabulation). Alcoholic delirium and brain damage are likely to come from thiamine deficiency. These last memory problems are somewhat common if brain is very low on acetylcholine.
Un-cited personal part: i recently noticed that almost all the unwanted side effects my sedative (pregabalin) causes overlapped with thiamine deficiency. I've experienced almost all previously mentioned temporary symptoms with that except serious memory problems. Pregabalin blocks calcium channels and it has mostly weak effect on all neurotransmitters (each is released after calcium triggers release). During minor withdrawal phase ~12 after dosing i often notice weird weakness and changes in posture with very foggy thinking with runny nose. Maybe it was because GABA and acetylcholine were being released too fast after calcium channel normalized and ran low so thiamine was diverted to producing those neurotransmitters and after awhile also run low causing deficiency symptoms. While inside cells neurotransmitters should preserve well but after releasing they get broken up often in split second. For example acetylcholine activates muscles controlled by willpower and this effect on muscles disappears fast because it gets broken up fast outside cells by acetylcholinesterase (each enzyme molecule breaking about 25 000 acetylcholine molecules per second). Acetylcholine is involved in nose mucus gland activation so anything causing that could cause problems with thiamine reserves. After noticing that it could explain why these outwardly visible symptoms happened i almost quit pregabalin overnight after taking it ~300 mg daily for over 3 years and got rid of these symptoms (surprising lack of withdrawals after 24 hour pause). I suspect most antipsychotics and sedatives could cause this effect on small scale but ethanol is still the most obvious cause for thiamine deficiency with worst magnitude that i know about. One nonacademic source listed diuretics, nicotine (binding with nicotinic acetylcholine receptors) and barbiturates (GABA receptor blockers) as substances that can adversely affect thiamine levels.
Tuesday, October 16, 2012
Floating gate transistors
Floating gate MOSFET transistors use relatively high voltage to force few electrons into "floating gate" that is surrounded by non-conductive material like glass. Such memory storage can preserve data for many years without any electricity source and these floating gates are common in memory cards and Flash memory sticks or other chip shaped storages. While good at keeping data for long time they are slow at writing as capacitors have to charge up around 5-12 volt charge to push electrons there and later similar voltage is needed to push that electron out of floating gate. Fast computer memories like DRAM or SRAM read-write fast using about 1-1,5 volts but they lose data fast and DRAM has to rewrite its contents several times each second. These DRAM and other unstabler RAM memories use capacitors to hold charge but they leak their charge and this leaking happens faster with smaller capacitors so future RAMs may need to rewrite increasingly faster.
Floating gates are filled with the help of control gate with varying charge that can pull or push electron to desired direction depending on if it used for writing, reading or erasing (last 2 are similar at first). Like other static electricity using memories that use electrons they lose their contents every time they are read because electrons get pushed out towards sensor and if data was detected in floating gate then it is usually automatically rewritten.
Wednesday, October 10, 2012
Electronegativity related to acids
Acids, similarly to other chemical reactions, tend to connect atoms that have largest difference between their electronegativities (EN). For example mixing NaOH and HCl in water creates NaCl and extra water. As strong acid HCl breaks up (disassociates) completely in water forming hydrogen and chlorine ions so probably all atoms in them combine into salt or water.
Strong acids like HCl, HI and HBr disassociate completely creating about same amount of hydrogen ions as there were strong acid molecules added to water. All these 3 simpler examples are pairs of atoms that have smaller difference in EN than H and O so if mixed with water then atoms in these acids tend to combine with water molecules leaving no intact acid molecule.
Weak acid like acetic acid in vinegar don't disassociate completely. Amount of hydrogen ions in vinegar is about 0,4% of the number of acetic acid molecules. This may be due to oxygen atoms so close together to region that loses hydrogen (-OH) like in other carboxylic acids. If hydrogen is released it would be attracted back to oxygen atoms.
Citric acid above. Many weak acids have such part with carbon connected to 2 oxygen atoms. Weak acids have central atoms with with more electronegativity than hydrogen.
Superacids can be over million time more acidic than strong acids. One consistent part of their structure is having many electronegative atoms outside connected to one or few atoms in middle which are less electronegative than hydrogen. Some superbases have opposite relation as they may have electronegative atom surrounded by less electronegative elements.
For example HSbF6 is the strongest known superacid over trillion times more acidic than sulfuric acid. Hydrogen ion is thought to keep moving between fluorine atoms due to very weak connections with main molecule so they are easily released into the mix.
Triflic acid.
Carborane. Green=chlorine, pink=boron, black=carbon and white is hydrogen.
Target atoms of acids could be predicted to some extent if EN is considered. Many organic molecules like starch and cellulose have ether bonds where oxygen atom is between 2 carbon atoms. EN difference between C and O is smaller than between H and O so in acidic mix these ether bonds break up leaving -OH groups to both carbons that used be connected with same oxygen atoms.
EN related to acid resistance
Hydrofluoric acid doesn't corrode some plastics, gold or silver but it does dissolve ceramics (ingredients include Al and Ca ), glass, iron, nickel, titanium and most other materials. While HF reacts with copper it doesn't react with nickel and many other elements with electronegativity over 1,9 but things are fuzzier around this 1,9 region. Elements with EN closer to EN of fluorine are usually more fluorine resistant.
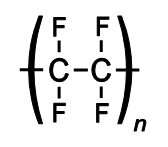
HF can be safe(ish)ly held in polyethylene (structure above) bottle while it could dissolve glass.
Other materials that can tolerate HF are Teflon (left) and neoprene (common synthetic rubber) to right.
Subscribe to:
Posts (Atom)