For those wondering how people could find out from what element something is made out of, knowing how mass spectrometry (MS) works could be the answer.
(image from here) Above illustration gives general idea about MS.
1) first sample is vaporised and electrically charged with electron beam. Depending on beams energy molecules could be broken into smaller molecules or in more energetic extreme every atom may be separated from each other.
2) electron beam removes electron giving atoms positive charge (all elements get positive charge with powerful enough electron beam). Positively charged electrode will capture electrons and push away positive ions.
3) moving ions reach magnetic field with magnetic field line perpendicular to ion trajectory. If a charged particle moves through magnetic field like that it will start to turn depending on pole positions and charge of particle. To achieve turn like in upper example with positive ions magnetic southern pole should be towards viewer and north pole on the other side of image.
4) more charged and lighter ions turn more and more massive ions turn less. Massive ions also take much more time to reach sensors. This technique also recognizes other isotopes because different neutron number affects the mass but not charge.
One co-inventor of MS was Joseph John Thompson (Nobel Prize winner for discovering electrons) who was also credited with discovering isotopes.
5) In the end ions could be photographed. In primitive versions simple photo plates were used. They react to visible light but also to charged energetic particles. Today semiconductor sensors similar to the ones used in digital cameras could be used to sense where the ions moved and turn it into a electric signal for monitor.
One of the earliest MS photos (source) showing the discovery of different neon isotopes.
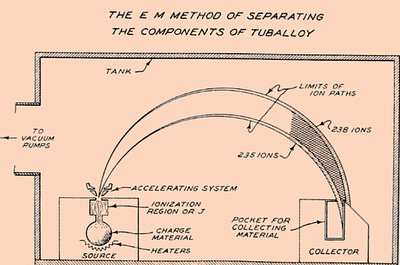
Calutron device was basically a mass spectrometer made for isotope separation for nuclear weapons. First large version was good enough to enrich uranium to 15% of U235. It was very energy consuming and got later replaced with cheaper and more productive methods.
Like typical MS it vaporized uranium with electron beam, accelerated it away from heat and magnetic field made ions turn in predictable way. Lighter U235 isotopes turned faster in magnetic field and reached collecting vessel while heavier U238 missed the vessel and got removed.



No comments:
Post a Comment