For those wondering how people could find out from what element something is made out of, knowing how mass spectrometry (MS) works could be the answer.
(image from here) Above illustration gives general idea about MS.
1) first sample is vaporised and electrically charged with electron beam. Depending on beams energy molecules could be broken into smaller molecules or in more energetic extreme every atom may be separated from each other.
2) electron beam removes electron giving atoms positive charge (all elements get positive charge with powerful enough electron beam). Positively charged electrode will capture electrons and push away positive ions.
3) moving ions reach magnetic field with magnetic field line perpendicular to ion trajectory. If a charged particle moves through magnetic field like that it will start to turn depending on pole positions and charge of particle. To achieve turn like in upper example with positive ions magnetic southern pole should be towards viewer and north pole on the other side of image.
4) more charged and lighter ions turn more and more massive ions turn less. Massive ions also take much more time to reach sensors. This technique also recognizes other isotopes because different neutron number affects the mass but not charge.
One co-inventor of MS was Joseph John Thompson (Nobel Prize winner for discovering electrons) who was also credited with discovering isotopes.
5) In the end ions could be photographed. In primitive versions simple photo plates were used. They react to visible light but also to charged energetic particles. Today semiconductor sensors similar to the ones used in digital cameras could be used to sense where the ions moved and turn it into a electric signal for monitor.
One of the earliest MS photos (source) showing the discovery of different neon isotopes.
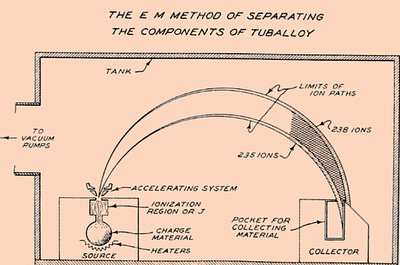
Calutron device was basically a mass spectrometer made for isotope separation for nuclear weapons. First large version was good enough to enrich uranium to 15% of U235. It was very energy consuming and got later replaced with cheaper and more productive methods.
Like typical MS it vaporized uranium with electron beam, accelerated it away from heat and magnetic field made ions turn in predictable way. Lighter U235 isotopes turned faster in magnetic field and reached collecting vessel while heavier U238 missed the vessel and got removed.
Thursday, April 26, 2012
How MRI and nuclear magnetic resonance (NMR) spectroscopy works
NMR is measured as radio frequency EM radiation from nuclei with odd number of protons and/or neutrons when they have been stimulated by similar frequency radio waves from some outside source so basically echoing radio waves. Such nuclei produce frequencies linearly dependent on how strong is the magnetic field on atoms. For example a 1 tesla magnetic field creates X frequency but 2 teslas produce 2 times higher frequency. If that nucleus has connection with other atoms its NMR frequency is altered by usually few parts in million. Normally in magnetic field of Earth hydrogen echos ~10 000-20 000 Hz radio waves but several Teslas strong fields can echo close to thousand megahertz or more which can make it easier to check 1 in million frequency changes to find molecular structure with radio waves without destroying the sample. In fMRIs for example they are commonly used to check where is oxygen used by the amount of hemoglobin without oxygen (with oxygen hemoglobin is nonmagnetic but without it it becomes magnetic so metabolism speed alters how much body part reflects/echos radio waves). Using several tesla NMR detectors could In everyday life hydrogen itself or radioactive isotopes of other elements show up in NMR.
In NMR spectroscopy you could use NMR to possibly find out from what chemical bonds some sample has by measuring mostly hydrogen signal. For example carbohydrate (with organic samples hydrogen atoms are usually main element that absorbs and echos radio waves) sample is put in a evenly strong magnetic field and many different radio frequencies are sent through it. Nuclei are sensitive to certain frequency in given magnetic field and after stopping artificial radio waves the same antenna could be used to measure radio waves from sample atoms for up to few seconds afterwards.
These measured waves are bit different depending if hydrogen is attached to other atom but to measure 1 in million differences strong magnetic field may be needed because the magnetic field of Earth gives hydrogen about 10 000-20 000 Hz radio frequency that is almost useless for measuring frequency changes around 1 in million.
MRI scanners measure almost only the hydrogen in body because almost all O, N an C has even number of nucleons (protons/neutrons) and don't have NMR signals. MRI (image source link) still uses NMR but with antennas from many angles.
Above image illustrates magnetic fields turned on from different sides. Different body areas get different magnetic field strength and therefore emit different radio frequencies (after absorbing same radio frequency). For example if you put your fingers near a magnet with different fingers at different distances from magnet then the MRI antenna should find 5 different frequency peaks for each finger (plus some broad signal range for rest of palm) but if fingers are in equal distance and in equal magnetic fields then only one peak may be visible. The emitted different radio frequencies are not absorbed by body and exit body to partly reach antennas. MRI machine tries out different angles for body and every time it calculates about how far from magnet was the hydrogen rich or poor area.
With stronger magnets it becomes easier to see tinier differences in hydrogen density.
In NMR spectroscopy you could use NMR to possibly find out from what chemical bonds some sample has by measuring mostly hydrogen signal. For example carbohydrate (with organic samples hydrogen atoms are usually main element that absorbs and echos radio waves) sample is put in a evenly strong magnetic field and many different radio frequencies are sent through it. Nuclei are sensitive to certain frequency in given magnetic field and after stopping artificial radio waves the same antenna could be used to measure radio waves from sample atoms for up to few seconds afterwards.
These measured waves are bit different depending if hydrogen is attached to other atom but to measure 1 in million differences strong magnetic field may be needed because the magnetic field of Earth gives hydrogen about 10 000-20 000 Hz radio frequency that is almost useless for measuring frequency changes around 1 in million.
MRI scanners measure almost only the hydrogen in body because almost all O, N an C has even number of nucleons (protons/neutrons) and don't have NMR signals. MRI (image source link) still uses NMR but with antennas from many angles.
Above image illustrates magnetic fields turned on from different sides. Different body areas get different magnetic field strength and therefore emit different radio frequencies (after absorbing same radio frequency). For example if you put your fingers near a magnet with different fingers at different distances from magnet then the MRI antenna should find 5 different frequency peaks for each finger (plus some broad signal range for rest of palm) but if fingers are in equal distance and in equal magnetic fields then only one peak may be visible. The emitted different radio frequencies are not absorbed by body and exit body to partly reach antennas. MRI machine tries out different angles for body and every time it calculates about how far from magnet was the hydrogen rich or poor area.
With stronger magnets it becomes easier to see tinier differences in hydrogen density.
Introduction
Unless I find other uses for this part I'll keep it short by saying from where I learned about these operating mechanisms- almost entirely from 2 pages:
wikipedia and http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
Subscribe to:
Posts (Atom)