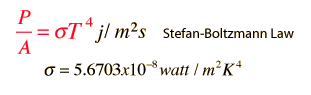In nature slime is mostly composed of fiber like proteins that are often covered by many glucose groups. Common type of mucus proteins in mammals is mucin and this is also present in fish slime. Mucins can have molecular mass of 1-10 million hydrogen atoms and in addition to being long fibers they also form many sulfur "bridges" between cysteine parts that connect random parts of fibers making it possible to connect several different mucin molecules with sulfur. Having glycose molecules attached makes mucin and other proteins likelier to attract water and avoid degradation by proteases.
One possible use for slime could be in making strong strands although as proteins they probably degrade if bacteria and humidity could touch it. Hagfishes produce lots of slime for self-defense (as shown in Hammonds Miracles of Nature 3 scene). After drying it turns into strong fiber close to the strength of spider silk although with less complex structure.
Prostate secretes some proteins that break up proteins making entire mixture more fluid. For example PSA (prostate-specific antigen) breaks up proteins that kept semen more solid and liquify it for ejaculating.
Slime produced by any body part is likely to smell "fishy" as they share exactly same odor molecule. One receptor that releases slime is acetylcholine and it eventually breaks down to trimethylamine which smells fishy and this could be felt in the mucus/slime of nose, mouth, vagina, penis (due to slime part of semen) and fishes.
Sunday, December 23, 2012
Wednesday, November 7, 2012
Magnetism and electron configuration
In short atoms and molecules are repelled by magnets (diamagnetic) if they have no unpaired electrons (only electrons with 2 different spins in orbitals) but are somewhat attracted to magnets (paramagnetic) when they have at least 1 unpaired (electron with 1 spin in orbital) electron in their outermost electron layer. Diamagnetism is weakest form of magnetism and due to this weakness it shows up usually when material has no unpaired electron. Also core electrons deeper in atom contribute to diamagnetism due to aligning with outside magnetism and causing internal magnetic field that pushes away from outside magnetic field. Diamagnetic response is believed to have similar mechanism to larger scale magnetic responses as outside magnetic field that can move electrons in material cause magnetic field in that material (superconductor, ring of wire, benzene ring or just atom) that tries to repel outside magnetic field.
Some of the more stronger room temperature diamagnets are purely carbon compounds like graphene, pyrolytic carbon and diamonds. Carbon has connection to 4 other atoms and in these materials all 4 are paired making all carbon atom electrons diamagnetic.
Water is weakly diamagnetic (superconductor are about 100 000 times more diamagnetic than water) and its stream can be pushed few millimeters away from strong magnet (clip). Another more sensitive way to test it is to put water container on floating vessel and slowly push it by holding magnet near water (clip). As animals are mostly water they can be floated above strong enough magnets. Frogs may float at around 10 tesla (clip). Humans have been tested in at least 7 tesla MRIs and while they didn't float they had some additional side effects like flashes after changing direction of magnetic field. These flashes were suspected to be caused by diamagnetic responses in parts of retinal rod cells that realign themselves and by pressing against each other they may activate each others. Rhodopsin is the light sensitive protein in rod cells and at least these proteins are also diamagnetic.
Copper is one of the few diamagnetic metals (clip) and copper pipes can slow down strong magnets falling through them.
Main difference between ferromagnets and paramagnets is that ferromagnets don't need outside magnetic field to become and stay magnetic. Both of these arise from unpaired electron but crystal structure of ferromagnets keeps them from losing magnetism due to heat movements that remove magnetism from paramagnetic materials with random movements. Permanent magnets use crystal structure that are magnetic in certain directions while resisting alignments in other directions. In production of permanent magnets all these crystals get aligned in one direction so entire material would spontaneously magnetize in same direction with enough stability to stay that way below curie temperatures.
Table showing unpaired electrons for single ions. Single hydrogen atom is purely paramagnetic and attracted to magnets but it becomes diamagnetic after becoming hydrogen molecule with all electrons paired. Transition metals have unfilled d orbitals that make them paramagnetic or ferromagnetic. Electron are in increasingly more diamagnetic configurations in lower rows of transition metals and most famous strong magnets use elements from 1st row of transition metals.
Electron pairing is usually shown with single or 2 opposite oriented arrows. Transition metals usually have electron orbitals with same energies (degenerate orbitals) and electrons can move freely as gas in transition metals between orbitals. Magnetism can arise in combination with other element. According to crystal field theory electrons stop having equal energy if other element has electrostatic effect on metal atom by pulling or pushing electrons to 1 side of atom.
2 rows show orbitals on side of metal closer to and further from other element that had electrostatic effect. Electrons closer to neighboring atom gets some extra energy from electrostatic interaction and if energy difference between orbitals is small then electrons can occupy them singly with high spin electrons (arrow up).
If resulting interaction between atoms makes some orbitals harder to access so it would take less energy to combine with other electron on same orbital then electrons tend to pair up (low-spin) and not go on new orbital unless all single electrons are paired.
Some of the more stronger room temperature diamagnets are purely carbon compounds like graphene, pyrolytic carbon and diamonds. Carbon has connection to 4 other atoms and in these materials all 4 are paired making all carbon atom electrons diamagnetic.
Water is weakly diamagnetic (superconductor are about 100 000 times more diamagnetic than water) and its stream can be pushed few millimeters away from strong magnet (clip). Another more sensitive way to test it is to put water container on floating vessel and slowly push it by holding magnet near water (clip). As animals are mostly water they can be floated above strong enough magnets. Frogs may float at around 10 tesla (clip). Humans have been tested in at least 7 tesla MRIs and while they didn't float they had some additional side effects like flashes after changing direction of magnetic field. These flashes were suspected to be caused by diamagnetic responses in parts of retinal rod cells that realign themselves and by pressing against each other they may activate each others. Rhodopsin is the light sensitive protein in rod cells and at least these proteins are also diamagnetic.
Copper is one of the few diamagnetic metals (clip) and copper pipes can slow down strong magnets falling through them.
Main difference between ferromagnets and paramagnets is that ferromagnets don't need outside magnetic field to become and stay magnetic. Both of these arise from unpaired electron but crystal structure of ferromagnets keeps them from losing magnetism due to heat movements that remove magnetism from paramagnetic materials with random movements. Permanent magnets use crystal structure that are magnetic in certain directions while resisting alignments in other directions. In production of permanent magnets all these crystals get aligned in one direction so entire material would spontaneously magnetize in same direction with enough stability to stay that way below curie temperatures.
Table showing unpaired electrons for single ions. Single hydrogen atom is purely paramagnetic and attracted to magnets but it becomes diamagnetic after becoming hydrogen molecule with all electrons paired. Transition metals have unfilled d orbitals that make them paramagnetic or ferromagnetic. Electron are in increasingly more diamagnetic configurations in lower rows of transition metals and most famous strong magnets use elements from 1st row of transition metals.
Electron pairing is usually shown with single or 2 opposite oriented arrows. Transition metals usually have electron orbitals with same energies (degenerate orbitals) and electrons can move freely as gas in transition metals between orbitals. Magnetism can arise in combination with other element. According to crystal field theory electrons stop having equal energy if other element has electrostatic effect on metal atom by pulling or pushing electrons to 1 side of atom.
2 rows show orbitals on side of metal closer to and further from other element that had electrostatic effect. Electrons closer to neighboring atom gets some extra energy from electrostatic interaction and if energy difference between orbitals is small then electrons can occupy them singly with high spin electrons (arrow up).
If resulting interaction between atoms makes some orbitals harder to access so it would take less energy to combine with other electron on same orbital then electrons tend to pair up (low-spin) and not go on new orbital unless all single electrons are paired.
Sunday, October 28, 2012
Electronegativity and reactivity of molecules
In general molecules are more stable if there is large difference between electronegativities of their atoms but less stable and more energetically reacting if they are made of atoms with similar electronegativites (EN) excluding those elements with 4 free electrons like carbon that can form strong cubical crystal structure.
For example most metals react with oxygen when they could come in contact with that. Also pure sodium and other alkali metals start burning in water but they lose reactivity after combining with chlorine or other element from opposite side of periodic table. While nitrogen doesn't react easily with organic materials it can react in contact with lithium or magnesium. EN difference of 1,5 or more is usually enough to start energetic (often flaming) chemical reaction without any need to add heat but with smaller differences heat or catalyst are needed like between carbon and oxygen.
Energy releasing metabolism and burning usually create molecules with larger difference of EN between its atoms than before. For example fats and sugars often have mainly carbon and hydrogen connected to each other which have 0,35 difference in EN but after turning them into CO2 (0,89) and water (1,2 difference) this difference grows and resulting molecules need much energy (heat or energetic electric current) to break those up.
Precious metals in middle columns are relatively nonreactive or resistant to most acids, oxygen and other reactive substances compared to other metals. Among the most stable metals are metals with EN at least 2,2 (or more) like gold and those with less EN can rust like silver but others between like Mo usually need heat to start combining with oxygen without visibly rusting in room temperature air.
Many infrared sensors use unstable molecules that create electron flow with even less energy than visible light provides. These are often cooled to 60-100 degrees above absolute zero to avoid detectors from blinding itself by creating electron currents due to room temperature and cooling is commonly needed to see room temperature heat. At the same time these same sensors without cooling are usually only useful for detecting temperatures that are hundreds of degrees above room temperature. As example PbSe has elements with EN differences of 0,2 and can detect infrared with 5-6 micrometer wavelength. Heat from human body is about 10 micrometer infrared radiation.
One of the most sensitive infrared sensor materials is mixture of Hg, Te and Cd which can detect almost 2-3 times less energetic infrared radiation than PbSe and it has about 2 times smaller difference in EN. Due to sensitivity HgTeCd has to be about 77 degrees above absolute zero to detect weaker infrared wavelength (up to ~12 micrometer wavelength for this mixture).
Thursday, October 18, 2012
Few comments about thiamine (vitamin B1) deficiency
Awareness about thiamine/vitamin B1 (other vitamin B types can run out together with B1) is maybe most important to those who use drugs that influence GABA and acetylcholine levels. GABA influencing drugs include ethanol, GHB and almost all sedatives like benzodiazepines and barbiturates. Acetylcholine is needed to move, remember stuff and to keep many glands working so most drugs that cause dryness in mouth, skin, mucus membranes and eyes probably block acetylcholine activity.
Thiamine is needed for at least 3 enzymes that produce energy from carbohydrates. It usually becomes effective for proteins after it gets 2 phosphate groups added to it. Alcohol stops thiamine absorption from stomach and also slows its activation by slower addition of phosphate groups (that last reaction is slowed because it needs magnesium and ethanol also lowers magnesium levels). While all cells seem to need it, heart and neurons are most sensitive. Serious lack of B1 can cause coma leading to death or deadly heart failure. It is also needed to produce GABA, acetylcholine and building materials to proteins, myelin and DNA plus many other substances. Usually thiamine levels become low if there are problems with absorbing it from intestines due to alcohol and drugs or due to health problems like chronic diarrhea, vomiting, stomach surgery or excessive urination with diuretics. Thiamine requirements are about 0,33 mg for each 1000 kcal of nutrients eaten. Meat is one main source of thiamine like also whole grains and brown rice. White rice on other hand cause lack of thiamine which is often called beriberi. Wernicke-Korsakoff syndrome is common name for this deficiency if caused by chronic alcoholism.
Common symptoms of thiamine deficiency: weakness, apathy, faster heart rate, weaker reflexes, unwanted eye movements, trouble breathing, possibly fluid in lungs related to heart problems, edema of lower legs and droopy eyelids. In case of Korsakoff syndrome (more extreme deficiency) it can cause problems with remembering past, learning new memories and may lead to memories of events that didn't happen (confabulation). Alcoholic delirium and brain damage are likely to come from thiamine deficiency. These last memory problems are somewhat common if brain is very low on acetylcholine.
Un-cited personal part: i recently noticed that almost all the unwanted side effects my sedative (pregabalin) causes overlapped with thiamine deficiency. I've experienced almost all previously mentioned temporary symptoms with that except serious memory problems. Pregabalin blocks calcium channels and it has mostly weak effect on all neurotransmitters (each is released after calcium triggers release). During minor withdrawal phase ~12 after dosing i often notice weird weakness and changes in posture with very foggy thinking with runny nose. Maybe it was because GABA and acetylcholine were being released too fast after calcium channel normalized and ran low so thiamine was diverted to producing those neurotransmitters and after awhile also run low causing deficiency symptoms. While inside cells neurotransmitters should preserve well but after releasing they get broken up often in split second. For example acetylcholine activates muscles controlled by willpower and this effect on muscles disappears fast because it gets broken up fast outside cells by acetylcholinesterase (each enzyme molecule breaking about 25 000 acetylcholine molecules per second). Acetylcholine is involved in nose mucus gland activation so anything causing that could cause problems with thiamine reserves. After noticing that it could explain why these outwardly visible symptoms happened i almost quit pregabalin overnight after taking it ~300 mg daily for over 3 years and got rid of these symptoms (surprising lack of withdrawals after 24 hour pause). I suspect most antipsychotics and sedatives could cause this effect on small scale but ethanol is still the most obvious cause for thiamine deficiency with worst magnitude that i know about. One nonacademic source listed diuretics, nicotine (binding with nicotinic acetylcholine receptors) and barbiturates (GABA receptor blockers) as substances that can adversely affect thiamine levels.
Thiamine is needed for at least 3 enzymes that produce energy from carbohydrates. It usually becomes effective for proteins after it gets 2 phosphate groups added to it. Alcohol stops thiamine absorption from stomach and also slows its activation by slower addition of phosphate groups (that last reaction is slowed because it needs magnesium and ethanol also lowers magnesium levels). While all cells seem to need it, heart and neurons are most sensitive. Serious lack of B1 can cause coma leading to death or deadly heart failure. It is also needed to produce GABA, acetylcholine and building materials to proteins, myelin and DNA plus many other substances. Usually thiamine levels become low if there are problems with absorbing it from intestines due to alcohol and drugs or due to health problems like chronic diarrhea, vomiting, stomach surgery or excessive urination with diuretics. Thiamine requirements are about 0,33 mg for each 1000 kcal of nutrients eaten. Meat is one main source of thiamine like also whole grains and brown rice. White rice on other hand cause lack of thiamine which is often called beriberi. Wernicke-Korsakoff syndrome is common name for this deficiency if caused by chronic alcoholism.
Common symptoms of thiamine deficiency: weakness, apathy, faster heart rate, weaker reflexes, unwanted eye movements, trouble breathing, possibly fluid in lungs related to heart problems, edema of lower legs and droopy eyelids. In case of Korsakoff syndrome (more extreme deficiency) it can cause problems with remembering past, learning new memories and may lead to memories of events that didn't happen (confabulation). Alcoholic delirium and brain damage are likely to come from thiamine deficiency. These last memory problems are somewhat common if brain is very low on acetylcholine.
Un-cited personal part: i recently noticed that almost all the unwanted side effects my sedative (pregabalin) causes overlapped with thiamine deficiency. I've experienced almost all previously mentioned temporary symptoms with that except serious memory problems. Pregabalin blocks calcium channels and it has mostly weak effect on all neurotransmitters (each is released after calcium triggers release). During minor withdrawal phase ~12 after dosing i often notice weird weakness and changes in posture with very foggy thinking with runny nose. Maybe it was because GABA and acetylcholine were being released too fast after calcium channel normalized and ran low so thiamine was diverted to producing those neurotransmitters and after awhile also run low causing deficiency symptoms. While inside cells neurotransmitters should preserve well but after releasing they get broken up often in split second. For example acetylcholine activates muscles controlled by willpower and this effect on muscles disappears fast because it gets broken up fast outside cells by acetylcholinesterase (each enzyme molecule breaking about 25 000 acetylcholine molecules per second). Acetylcholine is involved in nose mucus gland activation so anything causing that could cause problems with thiamine reserves. After noticing that it could explain why these outwardly visible symptoms happened i almost quit pregabalin overnight after taking it ~300 mg daily for over 3 years and got rid of these symptoms (surprising lack of withdrawals after 24 hour pause). I suspect most antipsychotics and sedatives could cause this effect on small scale but ethanol is still the most obvious cause for thiamine deficiency with worst magnitude that i know about. One nonacademic source listed diuretics, nicotine (binding with nicotinic acetylcholine receptors) and barbiturates (GABA receptor blockers) as substances that can adversely affect thiamine levels.
Tuesday, October 16, 2012
Floating gate transistors
Floating gate MOSFET transistors use relatively high voltage to force few electrons into "floating gate" that is surrounded by non-conductive material like glass. Such memory storage can preserve data for many years without any electricity source and these floating gates are common in memory cards and Flash memory sticks or other chip shaped storages. While good at keeping data for long time they are slow at writing as capacitors have to charge up around 5-12 volt charge to push electrons there and later similar voltage is needed to push that electron out of floating gate. Fast computer memories like DRAM or SRAM read-write fast using about 1-1,5 volts but they lose data fast and DRAM has to rewrite its contents several times each second. These DRAM and other unstabler RAM memories use capacitors to hold charge but they leak their charge and this leaking happens faster with smaller capacitors so future RAMs may need to rewrite increasingly faster.
Floating gates are filled with the help of control gate with varying charge that can pull or push electron to desired direction depending on if it used for writing, reading or erasing (last 2 are similar at first). Like other static electricity using memories that use electrons they lose their contents every time they are read because electrons get pushed out towards sensor and if data was detected in floating gate then it is usually automatically rewritten.
Wednesday, October 10, 2012
Electronegativity related to acids
Acids, similarly to other chemical reactions, tend to connect atoms that have largest difference between their electronegativities (EN). For example mixing NaOH and HCl in water creates NaCl and extra water. As strong acid HCl breaks up (disassociates) completely in water forming hydrogen and chlorine ions so probably all atoms in them combine into salt or water.
Strong acids like HCl, HI and HBr disassociate completely creating about same amount of hydrogen ions as there were strong acid molecules added to water. All these 3 simpler examples are pairs of atoms that have smaller difference in EN than H and O so if mixed with water then atoms in these acids tend to combine with water molecules leaving no intact acid molecule.
Weak acid like acetic acid in vinegar don't disassociate completely. Amount of hydrogen ions in vinegar is about 0,4% of the number of acetic acid molecules. This may be due to oxygen atoms so close together to region that loses hydrogen (-OH) like in other carboxylic acids. If hydrogen is released it would be attracted back to oxygen atoms.
Citric acid above. Many weak acids have such part with carbon connected to 2 oxygen atoms. Weak acids have central atoms with with more electronegativity than hydrogen.
Superacids can be over million time more acidic than strong acids. One consistent part of their structure is having many electronegative atoms outside connected to one or few atoms in middle which are less electronegative than hydrogen. Some superbases have opposite relation as they may have electronegative atom surrounded by less electronegative elements.
For example HSbF6 is the strongest known superacid over trillion times more acidic than sulfuric acid. Hydrogen ion is thought to keep moving between fluorine atoms due to very weak connections with main molecule so they are easily released into the mix.
Triflic acid.
Carborane. Green=chlorine, pink=boron, black=carbon and white is hydrogen.
Target atoms of acids could be predicted to some extent if EN is considered. Many organic molecules like starch and cellulose have ether bonds where oxygen atom is between 2 carbon atoms. EN difference between C and O is smaller than between H and O so in acidic mix these ether bonds break up leaving -OH groups to both carbons that used be connected with same oxygen atoms.
EN related to acid resistance
Hydrofluoric acid doesn't corrode some plastics, gold or silver but it does dissolve ceramics (ingredients include Al and Ca ), glass, iron, nickel, titanium and most other materials. While HF reacts with copper it doesn't react with nickel and many other elements with electronegativity over 1,9 but things are fuzzier around this 1,9 region. Elements with EN closer to EN of fluorine are usually more fluorine resistant.
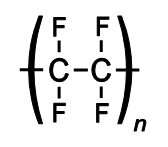
HF can be safe(ish)ly held in polyethylene (structure above) bottle while it could dissolve glass.
Other materials that can tolerate HF are Teflon (left) and neoprene (common synthetic rubber) to right.
Thursday, October 4, 2012
Relation between energy flow and electronegativity
In general materials are more conductive if they are made of same element so elements with same electronegativity are connected but non-conductive if electron current would have to switch from one element to other many times on the way. Also if they conduct electricity then they are probably good conductors of heat like metals. There are some exceptions as diamonds don't conduct electricity well (about 100 times better than glass) while being fast at conducing heat but this may be due to bit chaotic looking crystal structure (lack of straight pathways to flow to). Other pure carbon materials like graphite and nanotubes are good conductors and their simple structure allows more parallel flow of electrons.
Electrons are attracted to more electronegative elements and that could have predictable effect on electrical conductivity.
For example insulating quartz is made of silicon and oxygen connected one after other. If electron flows through it then it is bit attracted to every oxygen atom on the way unlike to silicon atoms around oxygen which lose electrons easily. Electrons could flow relatively easily from silicon to oxygen but not from that oxygen to any neighboring silicon atom so in large scale glass is about as conductive as air or vacuum. That may be the main reason why pure silicon is about billion to trillion times more conductive than glass.
Above are 4 examples of transparent conductive polymers which may be used in transmitting current through transparent materials like touch-screens. They also tend to have one type of element in main chain but there may be other elements in main chain like sulfur which has about 1% higher electronegativity than carbon. Possible that they are transparent while metals are reflective is due to only guiding energy in straight line not in every direction like in piece of metal. If light is polarized parallel to this chain it may more likely absorb but at same time allow light to pass if it is polarized in any other direction.
Many non-conductive polymers have oxygen atoms in main chain like polyester which can easily collect static electricity but there are several non-conductive polymers which don't have anything in main chain beside usual carbon and hydrogen.
Other special feature that these conductive polymers have is lack of charged parts next to main chain.
For example above structure is of non-conductive plexiglass. While main chain is carbon it has side chain that has oxygen atoms which could probably pull to the side any electrons that would move through main chain. Nylon has main chain with carbon and nitrogen but the the side it too has oxygen atoms that may interfere with its conductivity.
(Diode in above image) Electronegativity is in some ways used in electronics to create predictably routed flow of electrons by doping silicon with some element from group next to silicon. Adding aluminum (or other III A group element) would make it more positive (p-doped) and sulfur or arsenic from more electronegative 5th group can make it more negative (n-doped). 1 n-doped and 1 n-doped material connected together can create one way current in diodes. While electrons easily flow from negative area to more positive area, they may not flow at all in opposite direction or only if some high voltage pushed it against easy direction. Such setup is used in rectifiers to convert alternating current to direct current allowing only one way direction flow. Similar setup was used in cat whisker detectors in early radios that turned alternating current produced by absorbed radio waves in antenna into one way electricity.
LEDs use such diodes to create light or electricity. If electrons flowing in one direction get at least 1,2 electron volts of energy then they will start to glow. Same LED could create current if it was exposed to energetic enough photons that could get electrons moving (most likely only in one direction due to larger resistance in other direction).
Electrical conductivity is close to thermal conductivity as electrons carry energy/heat about as fast as wires could carry electricity. In this regard electronegativity could be used to predict how well and in what direction will materials carry energy/electricity/heat with the precision close to size of atoms involved. This precision could potentially be further increased by using magnetic fields to align atomic nuclei (and their electron movements) with outside magnetic fields or by smaller magnetic field produced by local groups of atoms. Cooling near absolute zero may further help to keep precision of interactions between atoms.
Electrons are attracted to more electronegative elements and that could have predictable effect on electrical conductivity.
For example insulating quartz is made of silicon and oxygen connected one after other. If electron flows through it then it is bit attracted to every oxygen atom on the way unlike to silicon atoms around oxygen which lose electrons easily. Electrons could flow relatively easily from silicon to oxygen but not from that oxygen to any neighboring silicon atom so in large scale glass is about as conductive as air or vacuum. That may be the main reason why pure silicon is about billion to trillion times more conductive than glass.
Above are 4 examples of transparent conductive polymers which may be used in transmitting current through transparent materials like touch-screens. They also tend to have one type of element in main chain but there may be other elements in main chain like sulfur which has about 1% higher electronegativity than carbon. Possible that they are transparent while metals are reflective is due to only guiding energy in straight line not in every direction like in piece of metal. If light is polarized parallel to this chain it may more likely absorb but at same time allow light to pass if it is polarized in any other direction.
Many non-conductive polymers have oxygen atoms in main chain like polyester which can easily collect static electricity but there are several non-conductive polymers which don't have anything in main chain beside usual carbon and hydrogen.
Other special feature that these conductive polymers have is lack of charged parts next to main chain.
For example above structure is of non-conductive plexiglass. While main chain is carbon it has side chain that has oxygen atoms which could probably pull to the side any electrons that would move through main chain. Nylon has main chain with carbon and nitrogen but the the side it too has oxygen atoms that may interfere with its conductivity.
(Diode in above image) Electronegativity is in some ways used in electronics to create predictably routed flow of electrons by doping silicon with some element from group next to silicon. Adding aluminum (or other III A group element) would make it more positive (p-doped) and sulfur or arsenic from more electronegative 5th group can make it more negative (n-doped). 1 n-doped and 1 n-doped material connected together can create one way current in diodes. While electrons easily flow from negative area to more positive area, they may not flow at all in opposite direction or only if some high voltage pushed it against easy direction. Such setup is used in rectifiers to convert alternating current to direct current allowing only one way direction flow. Similar setup was used in cat whisker detectors in early radios that turned alternating current produced by absorbed radio waves in antenna into one way electricity.
LEDs use such diodes to create light or electricity. If electrons flowing in one direction get at least 1,2 electron volts of energy then they will start to glow. Same LED could create current if it was exposed to energetic enough photons that could get electrons moving (most likely only in one direction due to larger resistance in other direction).
Electrical conductivity is close to thermal conductivity as electrons carry energy/heat about as fast as wires could carry electricity. In this regard electronegativity could be used to predict how well and in what direction will materials carry energy/electricity/heat with the precision close to size of atoms involved. This precision could potentially be further increased by using magnetic fields to align atomic nuclei (and their electron movements) with outside magnetic fields or by smaller magnetic field produced by local groups of atoms. Cooling near absolute zero may further help to keep precision of interactions between atoms.
Tuesday, October 2, 2012
About atomic structure of high-temperature superconductors
In general superconductors work at higher temperatures if they are made of more chemical elements. Superconductors that are made of one metallic element are usually superconductive in temperatures less than 10 degrees above absolute zero while high-temperature superconductors (HTS) working up to over 100 degrees above absolute zero may have 5 different elements. Copper oxides are present in HTSs that work at the highest known superconductive temperatures and for some time all known HTSs had copper although oxygen seems needed in all HTSs.
Crystal structure of such ceramics or alloys have mirroring layers that keep repeating through material. For example following 3 HTSs all have repeating layers with mirroring order.
TBCCO (thallium barium calcium copper oxide).
YBCO (yttrium barium copper oxide).
BSCCO (bismuth strontium calcium copper oxide).
This ordering may be suitable to create regular flow of electrons. Table of electronegativity can hint from where to where they'll flow. Calcium and barium have the lowest electronegativity among the above ingredients so they are most likely to lose electrons which flow to atoms that attract electrons stronger. Strongest attractors in TBCCO seems to be TlO layers (also labelled as "charge reservoirs" in above image) and BiO in BSCCO. Tl, Bi and O are all strong attractors of electrons although ionized calcium may eventually pull these electrons back. These layers provide somewhat flat few atom thick layers that move electrons easily. As these layers are so close to each others they may increase chances that electrons are always moving between layers due to pull between O and Ca or Ba. Superconductors levitate in magnetic field because they create magnetic field that opposes outside magnetic field. Charged particles in magnetic fields are forced to move circularly around magnetic field lines and in turn they themselves create magnetic field that opposes the outside magnetic field that caused circular movements for electrons and other charged particles. Having many charge reservoir layers every few atoms means that every microscopic part of HTS could locally react to outside magnetic field lines.
Crystal structure of such ceramics or alloys have mirroring layers that keep repeating through material. For example following 3 HTSs all have repeating layers with mirroring order.
TBCCO (thallium barium calcium copper oxide).
YBCO (yttrium barium copper oxide).
BSCCO (bismuth strontium calcium copper oxide).
This ordering may be suitable to create regular flow of electrons. Table of electronegativity can hint from where to where they'll flow. Calcium and barium have the lowest electronegativity among the above ingredients so they are most likely to lose electrons which flow to atoms that attract electrons stronger. Strongest attractors in TBCCO seems to be TlO layers (also labelled as "charge reservoirs" in above image) and BiO in BSCCO. Tl, Bi and O are all strong attractors of electrons although ionized calcium may eventually pull these electrons back. These layers provide somewhat flat few atom thick layers that move electrons easily. As these layers are so close to each others they may increase chances that electrons are always moving between layers due to pull between O and Ca or Ba. Superconductors levitate in magnetic field because they create magnetic field that opposes outside magnetic field. Charged particles in magnetic fields are forced to move circularly around magnetic field lines and in turn they themselves create magnetic field that opposes the outside magnetic field that caused circular movements for electrons and other charged particles. Having many charge reservoir layers every few atoms means that every microscopic part of HTS could locally react to outside magnetic field lines.
Catalase
Catalase is protein that turns H2O2 into water and oxygen molecules. It is present in almost all organisms and bacteria that could survive in oxygen but it is usually missing in species that don't tolerate oxygen like bacteria that live deeper underground or in the bottom of stagnant water.
In university biology class we each had to identify some bacteria by how they reacted to different substances. If bacteria tolerated oxygen then they started to bubble and foam after adding drop of hydrogen peroxide on them but lack of bubbles meant that those species couldn't survive well in oxygen. Same bubbling happens in larger organisms like in mushrooms, plants and animals. If wound is getting cleaned by H2O2 then it starts to bubble (not if it goes on undamaged skin). Likewise broken plant leaves would foam with H2O2 but not if it was added on unbroken leaves. Because body reacts this way to H2O2 it will also form bubbles deeper in body and also clog some small blood vessels near wound with small oxygen bubbles.
In university biology class we each had to identify some bacteria by how they reacted to different substances. If bacteria tolerated oxygen then they started to bubble and foam after adding drop of hydrogen peroxide on them but lack of bubbles meant that those species couldn't survive well in oxygen. Same bubbling happens in larger organisms like in mushrooms, plants and animals. If wound is getting cleaned by H2O2 then it starts to bubble (not if it goes on undamaged skin). Likewise broken plant leaves would foam with H2O2 but not if it was added on unbroken leaves. Because body reacts this way to H2O2 it will also form bubbles deeper in body and also clog some small blood vessels near wound with small oxygen bubbles.
Friday, September 14, 2012
Induction heating
If paramagnetic substance gets magnetized then it will warm little bit. Single magnetization doesn't warm so much that skin could sense difference as iron put against magnet can demonstrate but induction heaters use alternating currents with frequencies usually between 10-400 kHz so material can get magnetized in different directions over 800 000 times per second (in case of 400 kHz AC). Materials that magnetize easier warm up faster this way.
Such heater are built somewhat like inductors where wire is coiled around some metal which absorbs energy from wires as magnetic energy but "wires" in these heaters are very thick as they need lot of current for quick magnetization plus these copper wires are often fluid cooled. Magnetic energy that wires create depends equally on amperes and number of wire turns around material (ampere-turns).
Example of some attributes of this heater. While it uses high current it is somewhat safe to touch metal while it is being heated without getting electrocuted. He holds bare fingers against it for several seconds before he starts to move them away from glowing part. He later touched the wires which didn't get very hot. Only the part between wires seems to heat up and this 15 kW heater got that metal glowing in about 10 seconds.
Such heater are built somewhat like inductors where wire is coiled around some metal which absorbs energy from wires as magnetic energy but "wires" in these heaters are very thick as they need lot of current for quick magnetization plus these copper wires are often fluid cooled. Magnetic energy that wires create depends equally on amperes and number of wire turns around material (ampere-turns).
Friday, August 17, 2012
Food preservation with dehydration
Food dehydration can preserve food without for several years if it stays dry enough. One upside of this method is that it can preserve any solid foodstuff from sweet plants to raw or cooked meat. Although highly enough concentrated salt or sugar preserves food against decay they have the downside of affecting how the food tastes.
Although people have known for thousands of years that drying can preserve meat and other foodstuff they may still forget it when thinking about new foodstuff or about some specific company.
For example some McDonald's critics use logic that because their burgers don't rot or get moldy then they are supposedly full some scary preservatives or have no nutrition (even if they believe that its food makes people fatter). One McD burger had been kept for 14 years in room temperature without signs of rotting. Author of this article made his own burgers that he just dried out thoroughly enough. Small size of burgers made it easier to dry them but drying (article mentions ~93% water loss) could still take 3-7 days (depending on size) with the 73 C author knew about. Burgers without salt preserved about as well as salted ones. If burgers were kept in open air so they could absorb then McDonald's and homemade burgers both got moldy at same speed. If homemade humid burger was but in plastic bag with McD burger then within week they were both almost covered with mold.
Problem with water content is that it keeps enzymes/proteins working. Cells may die but proteins in this dead mass may keep breaking it up it there are any nutrients and large molecules left in this watery mix. That's one reason why pickles and homemade jams keep decaying no matter how thoroughly sterile they are. Proteins that build larger molecules mostly use energy so they can't function much after cell dies but the ones that break up glucose, fats, proteins and starch create energy in the process so old food keeps softening and losing nutrients with slow partial metabolism going on years after their cells died. On a small side note this relative independence of proteins from living cells means that if someone dies from alcohol or other drug overdose then their body would keep on breaking the drugs up if drug could be degraded by living body.
Proteins can be deactivated by high enough heat but that denaturation temperature can be hundreds of degrees. Simple and ancient way to deactivate proteins is to use drying as proteins need water to function. Proteins involved in metabolism don't seem to work in liquid cooking oil which also preserve well. Replacing water with cooking oil can give sense that food is humid (cooking oil is one way to make dried burgers feel soft, moist and juicy).
Thursday, July 26, 2012
Magnetic cooling and heating
Cooling by magnetic field can produce temperature of around 1 millionth of a degree over absolute zero. It works with paramagnetic substances which get magnetic only under the influence of outside magnetic field but not after external magnetic field is removed. If such materials are in magnetic field they heat up when their magnetic field lines up with outside magnetic field, then it gets cooled by coolant gas like liquid helium (boils at around 4 K or helium-3 that boils at around 0,5 K although exact boiling point depends a lot on pressure). After removing outside magnetic field material cools as its microscopic local magnetic field lines get back their random orientation and material stops being magnetic.
This effect was discovered on pure iron and cooling effect was ~0,5-2 kelvins per Tesla. Larger effect is achieved with alloys that have gadolinium metal which could cool 3-4 K per Tesla. Considering MRIs could have stable 3 Tesla magnetic field this could mean about 10 degree cooling per cooling cycle. Combined with helium cooling this could easily cool material below 1 K. This cooling doesn't work on all materials but cooled gadolinium pieces could be against the fall of room that is to be cooled.
Induction cooking uses this heating that happens during realignment of magnetic fields but without the cooling phase by quickly realigning magnetic fields with alternating current several times per second. As YouTube can show induction heating with enough power can make metal glow and melt within seconds.
Tuesday, July 24, 2012
Pressure of electromagnetic radiation
Electromagnetic radiation exerts pressure to everything that absorbs or reflects it. If 100% of EM radiation reflected then it would have twice as much pressure as it would have with 100% absorption.
This pressure of radiation (if all energy was absorbed and not reflected) in pascals is its power in watts per square meter divided by speed of light. Average solar radiation power per square meter is about 1370 W per square meter and causes about 4,6 micro pascals of pressure (like weight of 0,46 milligrams per square meter or twice that if all that reflected). 1 pascal is like 100 grams with earths gravity per 1 square meter and normal atmospheric pressure is about 10 tons per square meter.
This small pressure from sun is enough to make Nichols radiometers rotate. These use paddles with mirrors on one side for one way rotation and near vacuum environment to reduce air drag.
This pressure has been used to accelerate ions and small pieces of foil with powerful lasers. Circularly polarized light cause more even pressure. The laser power mentioned in this study had intensity of 1017−1022 W per square centimeters. They reached particle energies of up to 200 million electronvolts while in comparison about 10 000 electronvolts of energy is needed to create gamma ray photons. Very thin pieces of foil could accelerate in one piece.
1 watt laser can keep 5-10 micrometer particles hovering and with focus this laser could push with force that was 55 times more than the force from gravity on these particles.
Weak light pressure has been used to achieve coldest temperatures measured. In laser cooling devices light keep atoms suspended in middle of containers away from container walls that could transmit heat to atoms. With these devices temperature of around 1 billionth of a degree above absolute 0 have been achieved.
Pressure of radiation from hot objects
Stefan-Boltzmann law (source with calculator to find these values) describes among other things how many watts of power (P) per square meter (A) exit object with some temperature (T). Sigma value is constant. Increase in temperature increases power of heat radiation exponentially. 10 time difference in temperature causes 10 000 fold difference in energy of heat radiation. With higher power of heat radiation comes also faster cooling as heat exit it faster. Reflective objects emit less powerful radiation but this formula can give idea about how much energy could exit object in ideal circumstances.
Some results i got with previously mentioned formulas for 1 square meter area in all cases (reflective surfaces could have up to 2 times more pressure from these radiations):
In room temperature it radiates ~450 watts and causes pressure of ~1 micropascals.
100 C object could radiate 1090 watts and causes 3,6 micropascals of pressure.
800 K wood fire emits about 23 000 watts and causes 75 micropascals of pressure.
5800 K on surface of sun emits 64 megawatts per square meter (0,02 pascals).
150 000 K degree object radiates enough heat to cause pressure of 1 atmosphere (100 000 pascals).
50 000 000 degree nuclear explosion emits ~3,5×1023per square meter with pressure of ~1×1015 pascals that is comparable to 10 billion atmospheric pressures. That's like 100 billion tons or 100 cubic kilometers of water on 1 square meter of earth (if gravity was even for entire column). That pressure is enough to start nuclear fusion in thermonuclear weapons.
Tevatron particle accelerator creates up to ~1 trillion degree heat (Large Hadron Collider has achieved higher temperatures). If square meter could be heated to this temperature then the heat radiation could cause pressure of ~1,6×1032pascals which is ~1,6×1027 times atmospheric pressure. It's like 1,6×1031 kg in Earths gravity per square meter. Mass of Earth is ~5.9736×1024 kg and mass of sun is ~2×1030 kg so even mass of sun on 1 square meter may not be enough to overcome the pressure from 1 square meter 1 trillion degree heat.
Cooling with lower pressure
Increasing pressure of gas usually increases its temperature and reduction of pressure causes cooling in most gaseous substances except hydrogen, helium and neon at room temperature although at different temperatures they may cool during expansion. This effect is called Joule-Thomson effect. Gas canisters heat up during the time they get filled and they cool down to the temperature of surrounding environment but after decompressing it after that cooling they become much colder than they were before.
That's the effect that makes fridges, freezers and air conditioning devices work. They all compress coolant gas in one part of device and that makes it temporarily hotter but it gets to cool down to room or outside temperature on outside of device. If it gets to re-expand into larger tubes that surround for example inside of fridge then it will cool down to much lower temperature.
This effect also causes cooling of compressed spray deodorants and computer dusters (dusters can even cover surfaces with ice if they are sprayed upside down so the liquid could pour out).
Using the formula from there shows that reduction of air pressure by half decreases its absolute temperature by 20% which could cool average room temperature to around -35 C. 1% change in pressure should decrease it by ~1 C. 90% reduction in pressure decreases absolute temperature by half so room temperature would become about -120 C. Fast moving air vehicle part often cause air vortices and vortices have lower pressure in middle. That low pressure cooling condenses air vapor causing vapor stream on planes with also possible ice formation.


Diesel engines use this effect in reverse to ignite diesel by compressing air quickly to 40 atmospheres which heats it to 550 C and is enough to ignite diesel.
Sudden decompression could also be problem to submarines. Nuclear submarine USS Thresher
sank with 129 people as it could not rise to surface. It is suspected that while attempting to empty ballast water tank with compressed air it cooled so much it became clogged with ice and could not reduce its mass so it kept sinking until submarine couldn't take pressure and imploded. In testing with similar pressure they found that moisture in compressed air formed after few seconds of decompressing and that removing moisture from compressed air could avoid this problem.
Space shuttle engines can generate enough heat to vaporize it but they cool the engines by using liquid hydrogen as fuel and it cool down as depressurizing hydrogen cools it near 33 kelvins. Hydrogen tubes reach around engine and go inside were they spread through over thousand parallel metal pipes that don't melt due to cooling.
That's the effect that makes fridges, freezers and air conditioning devices work. They all compress coolant gas in one part of device and that makes it temporarily hotter but it gets to cool down to room or outside temperature on outside of device. If it gets to re-expand into larger tubes that surround for example inside of fridge then it will cool down to much lower temperature.
This effect also causes cooling of compressed spray deodorants and computer dusters (dusters can even cover surfaces with ice if they are sprayed upside down so the liquid could pour out).
Using the formula from there shows that reduction of air pressure by half decreases its absolute temperature by 20% which could cool average room temperature to around -35 C. 1% change in pressure should decrease it by ~1 C. 90% reduction in pressure decreases absolute temperature by half so room temperature would become about -120 C. Fast moving air vehicle part often cause air vortices and vortices have lower pressure in middle. That low pressure cooling condenses air vapor causing vapor stream on planes with also possible ice formation.


Diesel engines use this effect in reverse to ignite diesel by compressing air quickly to 40 atmospheres which heats it to 550 C and is enough to ignite diesel.
Sudden decompression could also be problem to submarines. Nuclear submarine USS Thresher
sank with 129 people as it could not rise to surface. It is suspected that while attempting to empty ballast water tank with compressed air it cooled so much it became clogged with ice and could not reduce its mass so it kept sinking until submarine couldn't take pressure and imploded. In testing with similar pressure they found that moisture in compressed air formed after few seconds of decompressing and that removing moisture from compressed air could avoid this problem.
Space shuttle engines can generate enough heat to vaporize it but they cool the engines by using liquid hydrogen as fuel and it cool down as depressurizing hydrogen cools it near 33 kelvins. Hydrogen tubes reach around engine and go inside were they spread through over thousand parallel metal pipes that don't melt due to cooling.
Monday, July 23, 2012
Basics of optics
In short materials and environments (air, vacuum, water, glass, diamonds) that don't conduct electricity are transparent if they don't have cracks or scrapes. Materials that conduct electricity (metals, graphite and pure silicon) are nontransparent and they can reflect light. For example thin metal foil can reflect light even if that traveled several light-years through space and atmosphere. In addition light reflects partially between areas that have different electric conductivity like between air and water or air and random nonconducting solid.
Cracks can make otherwise transparent materials like glass nontransparent by making it move semi-randomly in many directions. If light enters material with different refraction index and different local speed of light then it bends somewhat and cracks
For transparency material shouldn't have cracks and scrapes larger than the wavelength of visible light (around 0,5 micrometers).
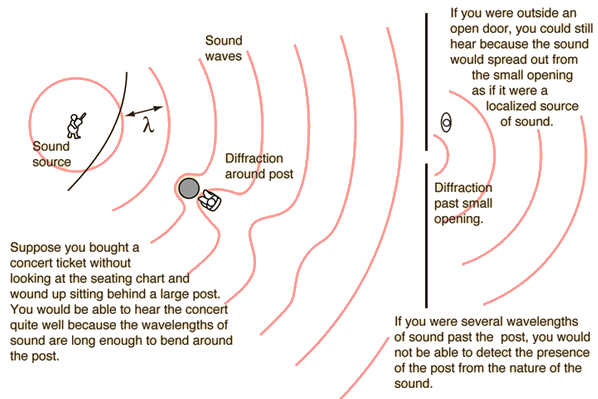
Light sound waves visible light waves also bend around things that are shorter than wavelength so within couple of wavelength waves straighten out and continue behind small objects. That's also the reason why radio waves used in radars have to be smaller than object to detect it and x-rays with wavelength similar to molecules can make molecular structure visible.
Cracks can make otherwise transparent materials like glass nontransparent by making it move semi-randomly in many directions. If light enters material with different refraction index and different local speed of light then it bends somewhat and cracks
For transparency material shouldn't have cracks and scrapes larger than the wavelength of visible light (around 0,5 micrometers).

Light sound waves visible light waves also bend around things that are shorter than wavelength so within couple of wavelength waves straighten out and continue behind small objects. That's also the reason why radio waves used in radars have to be smaller than object to detect it and x-rays with wavelength similar to molecules can make molecular structure visible.
Possibly useful HIV treatment by modifing bone marrow
HIV needs 2 specific receptors on T-cells to enter them and multiply. One is CD4 and other 2 are CCR5 and CXCR4. Those last 2 are receptors to chemokines that regulate movements of white blood cells. If those 2 are mutated enough then HIV can't enter them and it seems people with these mutations don't get infected with HIV without having serious health problems.
For example if all human ccr5 receptor genes have ccr5Δ32 mutation (32 base pairs missing) then they don't produce CCR5 and their T-cells don't get infected with R-5 (CCR5-tropic) HIV strain and those who have one mutated copy in one of the chromosome produce less CCR5 and tend to live longer after HIV infections. If HIV type uses CXCR4 then this previous mutation would not protect against infection.
Authors of this study decided to use proteins (zinc-finger nuclease) that break DNA from certain places so cxcr4 genes would mutate due to errors that happen when DNA parts rejoin. Using these proteins seemed to protect T-cells from HIV strains (X-4 tropic) that used CXCR4 for entering T-cells. If CCR5 and CXCR4 both got mutated enough then the protective effects improved.
Cells grown outside body seemed to tolerate these added mutations and kept multiplying.
Above illustration shows how much HIV affected different T-cells and how many were alive in different days. NTD is normal T-cell. R5ZFN is zinc finger nuclease that damages CCR5 and X4ZFN mutates CXCR4. X4ZFN seemed most protective against the 3 HIV strains they tested on T-cells without much cell death after HIV infection.
In one case a HIV positive patient got bone marrow due to leukemia and donor had ccr5Δ32 gene versions. Authors also noted that they know about humans with large CXCR4 mutations and that CXCR4 is involved in development of brain and cardiovascular system so mutation of that gene are probably deadly for fetus although mutations with ZFN in adults seem tolerable. As blood cells are produced in marrow it also caused production of mutated T-cells. After transplantation his HIV viral load remained undetectable even 3 years after transplantation.
Authors said it could be first "HIV cure" although transplantation of tissue from other person means patient has to take immune weakening drugs that may cause more serious infections and tumors. Also such mutations are rare for transplantations to all HIV positive patients. One alternative they offered was to use ZFN proteins to produce marrow with patients own cells that would produce HIV resistant T-cells.
My comment: if CXCR4 are needed to grow cardiovascular system and brain then mutations in them could make having children impossible if patients CXCR4 genes would get mutated in all their cells but authors wrote mostly about removing tissue, mutating it and re-transplanting.
Sunday, July 1, 2012
Strengthening materials
Materials such as ceramics, glasses and metals could be strengthened by adding extra chemical element that could form connections with as many atoms as possible. In case of chemical bonds that's with around 4 atoms and such elements are in middle of periodic table away from left and right edge.
Electrostatic attraction is other force that hold many materials together. Even hydrogen bond in water is example of electrostatic attraction and it helps with increasing melting and boiling temperatures.
Electronegativity shows how strongly some element attracts electrons. Substances with higher electronegativity usually get negative charge and the ones with smaller values get more positive charge in at least ionic bonds. That's partly because less electronegative elements have larger atoms and their electron are further away from positive charge in nucleus making these electrons easy to remove (giving atom positive charge) while in top right corner of periodic table atoms are smallest and positive charge of nucleus is closer to all its electrons so the strong attraction can give it extra electron(s).
Soft metals have very weak structure. For example sodium, lithium and other group 1 elements only have 1 electron for chemical bond so they could only form strong connection with 1 other atom and that makes these metals very soft and fragile. This lack of connection with other atoms may explain why these metals have low melting temperature (lithium melts at 180 C and sodium at 97 C).
One early example of material strengthening was in production of steel. Iron is fragile with too low or too high carbon % but if carbon forms 0,2-2,1% of steel by weight then this mixture can get extra strength. Usually at first carbon was burned out by pumping oxygen into melted iron mix to burn it away as CO2. After that people add required amount of carbon into molten metal in atmosphere that can't burn carbon like argon, helium and other noble gases.
These solutions may be common in production of other materials because often different elements get mixed in liquid hot environment where different ingredients melt and evaporate at different temperatures.
Carbon has more negative charge (more electronegative) than iron and that somewhat large difference in electronegativity can give steel strong electrostatic pull. Although oxygen and fluorine have higher electronegativity they have much less free electrons for forming bonds.
Iron has cubic body centered crystal structure that has 9 iron atoms in crystal unit. In case of 2% carbon it would mean about 10% of atoms are carbon and that makes about 1 carbon atom for each such cubical unit in iron crystal although iron could also have structure shown in middle.
In case of clay and porcelain diversity of ingredients can also help with durability.
Bone china is one more historic type of porcelain that Europeans learned to produce. One recipe for it is to mix kaolinite (25%), feldspar (25%) and bone ash (50%). Kaolinite (Al2S2O5(OH)4) is basically clean clay. Feldspar has also aluminium, silicon, oxygen and hydrogen but in addition sodium, calcium and potassium. Bone ash is left after burning bones and that composes mostly of phosphate groups and calcium (CaHPO4).
Special thing about bone ash is that both phosphate and calcium have double charges. Calcium has +2 charge and phosphate group has -2 charge making electrostatic with them extra strong and porcelain was partly valued because it resisted drops to floor better than clay.
Elements found in clay can also be used to produce very strong and scratch resistant transparent glass (porcelain is also somewhat transparent). One version of this strong glass has brand name Gorilla Glass and it used on newer more expensive phones and tablets. It is made from aluminum, silicon and oxygen like clay with additional potassium and sodium. After glass gets made from Al, Si, O and Na it gets strengthened by putting it in molten potassium salt at 400 C. Smaller sodium ions leave glass and larger potassium moves into glass that expanded during heating. After cooling glass constricts again and forces atoms tighter together but now atom are more densely packed due to larger potassium with larger differences in electronegativity between elements (and stronger electrostatic attraction) than before.
Example of Gorilla Glass in use.
Monday, June 25, 2012
Simple electric generators and motors
Above example of wire between 2 magnets could be used to make simpler direct current (DC) generator or DC motor. It should be easy enough to teach 10 year old kids in poorest countries how to build simple motors within hour. Batteries are source of DC.
Like seen on above image wires are close but not touching.
Alternating current (AC) using motors and generators can be similar but with extra complexity designed to rotate parts of it right amount before current changes direction so inertia would not be lost.
Reason how same design can work as generator and engine is that charged particles behave according to right hand rule. According to that if charged particle moves perpendicular to magnetic field lines (B) between south and north pole then magnetic field forces charged particles to move circularly in direction that is at 90 angle with present direction of current.
For example electron beam above keeps rotating in magnetic field with consistent radius. This effect is also used in particle accelerator sensors to see what charges did the new seen particles have as they know direction of magnetic field and which charge should rotate which way.
If charged particle moves perpendicular to magnetic field lines then they will always spiral or move circularly. Positive charge would be forced to move in direction F on above image and electron would turn to opposite side but if charge doesn't move in relation to magnet then they have no effect on each other.
These 3 directions B, v and F are at 90 degree angle with other 2 directions.

These 3 directions have been described with Fleming's left hand and right hand rule although they show same basic thing in different situations. Left hand example is used to describe generators and right hand example to illustrate engines. In left hand example if current flows in direction shown compared to magnetic field direction then entire wire would be pulled somewhat sideways (thrust of motion). In motor charges moved because electricity flowed but in generator this movement can be cause by moving wire or any electric conductor through magnetic field and charged particles would start to move but in generators wire is oriented so force makes charged particles move in wire not push wire sideways because charges couldn't exit wire through insulation. For example if wire is held parallel to direction F in third hand rule illustration and moved horizontally up towards v then magnetic field forces current to move in direction F. In motor if wire is vertical with direction v then letting current flow in that direction would force entire wire towards direction F if positive charge moved there but wire would be forced to opposite direction if electron moved towards direction v.
In above image arrows show direction of electron movements. Using bent wire that goes both ways allows different parts of wires to be forced in opposite directions keeping entire wire moving circularly.
Tips of wires are connected to different pieces of split rings. Carbon brushes transmit electricity to split ring and wire that connects its sides but because the gap of split-ring may lose connection with brushes every 180 degree turn so voltage can go near zero twice per turn.
In AC generator and motor these rings get used bit differently. Here different tips of bent wires get connected to different rings which change polarity regularly so generator or motor could keep turning the same way. Such motors should have stable AC frequency dependent rotating speeds while DC motors may be able to rotate with almost any speed that their strength and air resistance would allow.
Alternator is generator for alternating current. It doesn't matter much if magnets do the moving or wires as long conductors change location in magnetic field. Alternator causes electricity by using same physics phenomena but due to magnetic field reversing during this movement the current it produces is also reversed about as often as it takes for a 180 degree turn for magnet. As main bonus AC can be transported up to hundreds of kilometers with little loss in power and AC is required for radio waves with radio waves having same frequency as AC that flowed in antenna. High frequency radio waves needed to transmit TV channels and radio can't use a magnet rotating over 100 million times per second but electronically such AC frequencies can me made without moving parts using logic gates and central clock.
Thursday, April 26, 2012
Mass spectrometry
For those wondering how people could find out from what element something is made out of, knowing how mass spectrometry (MS) works could be the answer.
(image from here) Above illustration gives general idea about MS.
1) first sample is vaporised and electrically charged with electron beam. Depending on beams energy molecules could be broken into smaller molecules or in more energetic extreme every atom may be separated from each other.
2) electron beam removes electron giving atoms positive charge (all elements get positive charge with powerful enough electron beam). Positively charged electrode will capture electrons and push away positive ions.
3) moving ions reach magnetic field with magnetic field line perpendicular to ion trajectory. If a charged particle moves through magnetic field like that it will start to turn depending on pole positions and charge of particle. To achieve turn like in upper example with positive ions magnetic southern pole should be towards viewer and north pole on the other side of image.
4) more charged and lighter ions turn more and more massive ions turn less. Massive ions also take much more time to reach sensors. This technique also recognizes other isotopes because different neutron number affects the mass but not charge.
One co-inventor of MS was Joseph John Thompson (Nobel Prize winner for discovering electrons) who was also credited with discovering isotopes.
5) In the end ions could be photographed. In primitive versions simple photo plates were used. They react to visible light but also to charged energetic particles. Today semiconductor sensors similar to the ones used in digital cameras could be used to sense where the ions moved and turn it into a electric signal for monitor.
One of the earliest MS photos (source) showing the discovery of different neon isotopes.
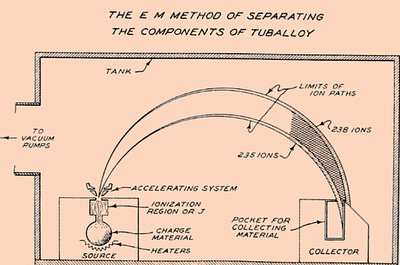
Calutron device was basically a mass spectrometer made for isotope separation for nuclear weapons. First large version was good enough to enrich uranium to 15% of U235. It was very energy consuming and got later replaced with cheaper and more productive methods.
Like typical MS it vaporized uranium with electron beam, accelerated it away from heat and magnetic field made ions turn in predictable way. Lighter U235 isotopes turned faster in magnetic field and reached collecting vessel while heavier U238 missed the vessel and got removed.
(image from here) Above illustration gives general idea about MS.
1) first sample is vaporised and electrically charged with electron beam. Depending on beams energy molecules could be broken into smaller molecules or in more energetic extreme every atom may be separated from each other.
2) electron beam removes electron giving atoms positive charge (all elements get positive charge with powerful enough electron beam). Positively charged electrode will capture electrons and push away positive ions.
3) moving ions reach magnetic field with magnetic field line perpendicular to ion trajectory. If a charged particle moves through magnetic field like that it will start to turn depending on pole positions and charge of particle. To achieve turn like in upper example with positive ions magnetic southern pole should be towards viewer and north pole on the other side of image.
4) more charged and lighter ions turn more and more massive ions turn less. Massive ions also take much more time to reach sensors. This technique also recognizes other isotopes because different neutron number affects the mass but not charge.
One co-inventor of MS was Joseph John Thompson (Nobel Prize winner for discovering electrons) who was also credited with discovering isotopes.
5) In the end ions could be photographed. In primitive versions simple photo plates were used. They react to visible light but also to charged energetic particles. Today semiconductor sensors similar to the ones used in digital cameras could be used to sense where the ions moved and turn it into a electric signal for monitor.
One of the earliest MS photos (source) showing the discovery of different neon isotopes.
Calutron device was basically a mass spectrometer made for isotope separation for nuclear weapons. First large version was good enough to enrich uranium to 15% of U235. It was very energy consuming and got later replaced with cheaper and more productive methods.
Like typical MS it vaporized uranium with electron beam, accelerated it away from heat and magnetic field made ions turn in predictable way. Lighter U235 isotopes turned faster in magnetic field and reached collecting vessel while heavier U238 missed the vessel and got removed.
How MRI and nuclear magnetic resonance (NMR) spectroscopy works
NMR is measured as radio frequency EM radiation from nuclei with odd number of protons and/or neutrons when they have been stimulated by similar frequency radio waves from some outside source so basically echoing radio waves. Such nuclei produce frequencies linearly dependent on how strong is the magnetic field on atoms. For example a 1 tesla magnetic field creates X frequency but 2 teslas produce 2 times higher frequency. If that nucleus has connection with other atoms its NMR frequency is altered by usually few parts in million. Normally in magnetic field of Earth hydrogen echos ~10 000-20 000 Hz radio waves but several Teslas strong fields can echo close to thousand megahertz or more which can make it easier to check 1 in million frequency changes to find molecular structure with radio waves without destroying the sample. In fMRIs for example they are commonly used to check where is oxygen used by the amount of hemoglobin without oxygen (with oxygen hemoglobin is nonmagnetic but without it it becomes magnetic so metabolism speed alters how much body part reflects/echos radio waves). Using several tesla NMR detectors could In everyday life hydrogen itself or radioactive isotopes of other elements show up in NMR.
In NMR spectroscopy you could use NMR to possibly find out from what chemical bonds some sample has by measuring mostly hydrogen signal. For example carbohydrate (with organic samples hydrogen atoms are usually main element that absorbs and echos radio waves) sample is put in a evenly strong magnetic field and many different radio frequencies are sent through it. Nuclei are sensitive to certain frequency in given magnetic field and after stopping artificial radio waves the same antenna could be used to measure radio waves from sample atoms for up to few seconds afterwards.
These measured waves are bit different depending if hydrogen is attached to other atom but to measure 1 in million differences strong magnetic field may be needed because the magnetic field of Earth gives hydrogen about 10 000-20 000 Hz radio frequency that is almost useless for measuring frequency changes around 1 in million.
MRI scanners measure almost only the hydrogen in body because almost all O, N an C has even number of nucleons (protons/neutrons) and don't have NMR signals. MRI (image source link) still uses NMR but with antennas from many angles.
Above image illustrates magnetic fields turned on from different sides. Different body areas get different magnetic field strength and therefore emit different radio frequencies (after absorbing same radio frequency). For example if you put your fingers near a magnet with different fingers at different distances from magnet then the MRI antenna should find 5 different frequency peaks for each finger (plus some broad signal range for rest of palm) but if fingers are in equal distance and in equal magnetic fields then only one peak may be visible. The emitted different radio frequencies are not absorbed by body and exit body to partly reach antennas. MRI machine tries out different angles for body and every time it calculates about how far from magnet was the hydrogen rich or poor area.
With stronger magnets it becomes easier to see tinier differences in hydrogen density.
In NMR spectroscopy you could use NMR to possibly find out from what chemical bonds some sample has by measuring mostly hydrogen signal. For example carbohydrate (with organic samples hydrogen atoms are usually main element that absorbs and echos radio waves) sample is put in a evenly strong magnetic field and many different radio frequencies are sent through it. Nuclei are sensitive to certain frequency in given magnetic field and after stopping artificial radio waves the same antenna could be used to measure radio waves from sample atoms for up to few seconds afterwards.
These measured waves are bit different depending if hydrogen is attached to other atom but to measure 1 in million differences strong magnetic field may be needed because the magnetic field of Earth gives hydrogen about 10 000-20 000 Hz radio frequency that is almost useless for measuring frequency changes around 1 in million.
MRI scanners measure almost only the hydrogen in body because almost all O, N an C has even number of nucleons (protons/neutrons) and don't have NMR signals. MRI (image source link) still uses NMR but with antennas from many angles.
Above image illustrates magnetic fields turned on from different sides. Different body areas get different magnetic field strength and therefore emit different radio frequencies (after absorbing same radio frequency). For example if you put your fingers near a magnet with different fingers at different distances from magnet then the MRI antenna should find 5 different frequency peaks for each finger (plus some broad signal range for rest of palm) but if fingers are in equal distance and in equal magnetic fields then only one peak may be visible. The emitted different radio frequencies are not absorbed by body and exit body to partly reach antennas. MRI machine tries out different angles for body and every time it calculates about how far from magnet was the hydrogen rich or poor area.
With stronger magnets it becomes easier to see tinier differences in hydrogen density.
Introduction
Unless I find other uses for this part I'll keep it short by saying from where I learned about these operating mechanisms- almost entirely from 2 pages:
wikipedia and http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
Subscribe to:
Posts (Atom)