Wednesday, October 10, 2012
Electronegativity related to acids
Acids, similarly to other chemical reactions, tend to connect atoms that have largest difference between their electronegativities (EN). For example mixing NaOH and HCl in water creates NaCl and extra water. As strong acid HCl breaks up (disassociates) completely in water forming hydrogen and chlorine ions so probably all atoms in them combine into salt or water.
Strong acids like HCl, HI and HBr disassociate completely creating about same amount of hydrogen ions as there were strong acid molecules added to water. All these 3 simpler examples are pairs of atoms that have smaller difference in EN than H and O so if mixed with water then atoms in these acids tend to combine with water molecules leaving no intact acid molecule.
Weak acid like acetic acid in vinegar don't disassociate completely. Amount of hydrogen ions in vinegar is about 0,4% of the number of acetic acid molecules. This may be due to oxygen atoms so close together to region that loses hydrogen (-OH) like in other carboxylic acids. If hydrogen is released it would be attracted back to oxygen atoms.
Citric acid above. Many weak acids have such part with carbon connected to 2 oxygen atoms. Weak acids have central atoms with with more electronegativity than hydrogen.
Superacids can be over million time more acidic than strong acids. One consistent part of their structure is having many electronegative atoms outside connected to one or few atoms in middle which are less electronegative than hydrogen. Some superbases have opposite relation as they may have electronegative atom surrounded by less electronegative elements.
For example HSbF6 is the strongest known superacid over trillion times more acidic than sulfuric acid. Hydrogen ion is thought to keep moving between fluorine atoms due to very weak connections with main molecule so they are easily released into the mix.
Triflic acid.
Carborane. Green=chlorine, pink=boron, black=carbon and white is hydrogen.
Target atoms of acids could be predicted to some extent if EN is considered. Many organic molecules like starch and cellulose have ether bonds where oxygen atom is between 2 carbon atoms. EN difference between C and O is smaller than between H and O so in acidic mix these ether bonds break up leaving -OH groups to both carbons that used be connected with same oxygen atoms.
EN related to acid resistance
Hydrofluoric acid doesn't corrode some plastics, gold or silver but it does dissolve ceramics (ingredients include Al and Ca ), glass, iron, nickel, titanium and most other materials. While HF reacts with copper it doesn't react with nickel and many other elements with electronegativity over 1,9 but things are fuzzier around this 1,9 region. Elements with EN closer to EN of fluorine are usually more fluorine resistant.
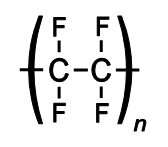
HF can be safe(ish)ly held in polyethylene (structure above) bottle while it could dissolve glass.
Other materials that can tolerate HF are Teflon (left) and neoprene (common synthetic rubber) to right.
Subscribe to:
Post Comments (Atom)








Can you tell me Why He is place in p block element
ReplyDeletePartly for having unreactive properties like other noble gases and like noble gases outer electron layer has no "occupancies" for other atoms to attach to. Otherwise it would make sense having it in s block due to electron configuration but maybe it got classified by different standards as it is very different from reactive s block elements.
DeleteIn case you need to know that for schoolwork i'd advise you ask teachers opinion where it belongs. Some authors have considered helium a s block element.
Delete