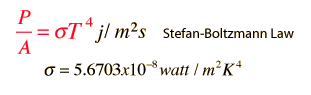Thursday, July 26, 2012
Magnetic cooling and heating
Cooling by magnetic field can produce temperature of around 1 millionth of a degree over absolute zero. It works with paramagnetic substances which get magnetic only under the influence of outside magnetic field but not after external magnetic field is removed. If such materials are in magnetic field they heat up when their magnetic field lines up with outside magnetic field, then it gets cooled by coolant gas like liquid helium (boils at around 4 K or helium-3 that boils at around 0,5 K although exact boiling point depends a lot on pressure). After removing outside magnetic field material cools as its microscopic local magnetic field lines get back their random orientation and material stops being magnetic.
This effect was discovered on pure iron and cooling effect was ~0,5-2 kelvins per Tesla. Larger effect is achieved with alloys that have gadolinium metal which could cool 3-4 K per Tesla. Considering MRIs could have stable 3 Tesla magnetic field this could mean about 10 degree cooling per cooling cycle. Combined with helium cooling this could easily cool material below 1 K. This cooling doesn't work on all materials but cooled gadolinium pieces could be against the fall of room that is to be cooled.
Induction cooking uses this heating that happens during realignment of magnetic fields but without the cooling phase by quickly realigning magnetic fields with alternating current several times per second. As YouTube can show induction heating with enough power can make metal glow and melt within seconds.
Tuesday, July 24, 2012
Pressure of electromagnetic radiation
Electromagnetic radiation exerts pressure to everything that absorbs or reflects it. If 100% of EM radiation reflected then it would have twice as much pressure as it would have with 100% absorption.
This pressure of radiation (if all energy was absorbed and not reflected) in pascals is its power in watts per square meter divided by speed of light. Average solar radiation power per square meter is about 1370 W per square meter and causes about 4,6 micro pascals of pressure (like weight of 0,46 milligrams per square meter or twice that if all that reflected). 1 pascal is like 100 grams with earths gravity per 1 square meter and normal atmospheric pressure is about 10 tons per square meter.
This small pressure from sun is enough to make Nichols radiometers rotate. These use paddles with mirrors on one side for one way rotation and near vacuum environment to reduce air drag.
This pressure has been used to accelerate ions and small pieces of foil with powerful lasers. Circularly polarized light cause more even pressure. The laser power mentioned in this study had intensity of 1017−1022 W per square centimeters. They reached particle energies of up to 200 million electronvolts while in comparison about 10 000 electronvolts of energy is needed to create gamma ray photons. Very thin pieces of foil could accelerate in one piece.
1 watt laser can keep 5-10 micrometer particles hovering and with focus this laser could push with force that was 55 times more than the force from gravity on these particles.
Weak light pressure has been used to achieve coldest temperatures measured. In laser cooling devices light keep atoms suspended in middle of containers away from container walls that could transmit heat to atoms. With these devices temperature of around 1 billionth of a degree above absolute 0 have been achieved.
Pressure of radiation from hot objects
Stefan-Boltzmann law (source with calculator to find these values) describes among other things how many watts of power (P) per square meter (A) exit object with some temperature (T). Sigma value is constant. Increase in temperature increases power of heat radiation exponentially. 10 time difference in temperature causes 10 000 fold difference in energy of heat radiation. With higher power of heat radiation comes also faster cooling as heat exit it faster. Reflective objects emit less powerful radiation but this formula can give idea about how much energy could exit object in ideal circumstances.
Some results i got with previously mentioned formulas for 1 square meter area in all cases (reflective surfaces could have up to 2 times more pressure from these radiations):
In room temperature it radiates ~450 watts and causes pressure of ~1 micropascals.
100 C object could radiate 1090 watts and causes 3,6 micropascals of pressure.
800 K wood fire emits about 23 000 watts and causes 75 micropascals of pressure.
5800 K on surface of sun emits 64 megawatts per square meter (0,02 pascals).
150 000 K degree object radiates enough heat to cause pressure of 1 atmosphere (100 000 pascals).
50 000 000 degree nuclear explosion emits ~3,5×1023per square meter with pressure of ~1×1015 pascals that is comparable to 10 billion atmospheric pressures. That's like 100 billion tons or 100 cubic kilometers of water on 1 square meter of earth (if gravity was even for entire column). That pressure is enough to start nuclear fusion in thermonuclear weapons.
Tevatron particle accelerator creates up to ~1 trillion degree heat (Large Hadron Collider has achieved higher temperatures). If square meter could be heated to this temperature then the heat radiation could cause pressure of ~1,6×1032pascals which is ~1,6×1027 times atmospheric pressure. It's like 1,6×1031 kg in Earths gravity per square meter. Mass of Earth is ~5.9736×1024 kg and mass of sun is ~2×1030 kg so even mass of sun on 1 square meter may not be enough to overcome the pressure from 1 square meter 1 trillion degree heat.
Cooling with lower pressure
Increasing pressure of gas usually increases its temperature and reduction of pressure causes cooling in most gaseous substances except hydrogen, helium and neon at room temperature although at different temperatures they may cool during expansion. This effect is called Joule-Thomson effect. Gas canisters heat up during the time they get filled and they cool down to the temperature of surrounding environment but after decompressing it after that cooling they become much colder than they were before.
That's the effect that makes fridges, freezers and air conditioning devices work. They all compress coolant gas in one part of device and that makes it temporarily hotter but it gets to cool down to room or outside temperature on outside of device. If it gets to re-expand into larger tubes that surround for example inside of fridge then it will cool down to much lower temperature.
This effect also causes cooling of compressed spray deodorants and computer dusters (dusters can even cover surfaces with ice if they are sprayed upside down so the liquid could pour out).
Using the formula from there shows that reduction of air pressure by half decreases its absolute temperature by 20% which could cool average room temperature to around -35 C. 1% change in pressure should decrease it by ~1 C. 90% reduction in pressure decreases absolute temperature by half so room temperature would become about -120 C. Fast moving air vehicle part often cause air vortices and vortices have lower pressure in middle. That low pressure cooling condenses air vapor causing vapor stream on planes with also possible ice formation.


Diesel engines use this effect in reverse to ignite diesel by compressing air quickly to 40 atmospheres which heats it to 550 C and is enough to ignite diesel.
Sudden decompression could also be problem to submarines. Nuclear submarine USS Thresher
sank with 129 people as it could not rise to surface. It is suspected that while attempting to empty ballast water tank with compressed air it cooled so much it became clogged with ice and could not reduce its mass so it kept sinking until submarine couldn't take pressure and imploded. In testing with similar pressure they found that moisture in compressed air formed after few seconds of decompressing and that removing moisture from compressed air could avoid this problem.
Space shuttle engines can generate enough heat to vaporize it but they cool the engines by using liquid hydrogen as fuel and it cool down as depressurizing hydrogen cools it near 33 kelvins. Hydrogen tubes reach around engine and go inside were they spread through over thousand parallel metal pipes that don't melt due to cooling.
That's the effect that makes fridges, freezers and air conditioning devices work. They all compress coolant gas in one part of device and that makes it temporarily hotter but it gets to cool down to room or outside temperature on outside of device. If it gets to re-expand into larger tubes that surround for example inside of fridge then it will cool down to much lower temperature.
This effect also causes cooling of compressed spray deodorants and computer dusters (dusters can even cover surfaces with ice if they are sprayed upside down so the liquid could pour out).
Using the formula from there shows that reduction of air pressure by half decreases its absolute temperature by 20% which could cool average room temperature to around -35 C. 1% change in pressure should decrease it by ~1 C. 90% reduction in pressure decreases absolute temperature by half so room temperature would become about -120 C. Fast moving air vehicle part often cause air vortices and vortices have lower pressure in middle. That low pressure cooling condenses air vapor causing vapor stream on planes with also possible ice formation.


Diesel engines use this effect in reverse to ignite diesel by compressing air quickly to 40 atmospheres which heats it to 550 C and is enough to ignite diesel.
Sudden decompression could also be problem to submarines. Nuclear submarine USS Thresher
sank with 129 people as it could not rise to surface. It is suspected that while attempting to empty ballast water tank with compressed air it cooled so much it became clogged with ice and could not reduce its mass so it kept sinking until submarine couldn't take pressure and imploded. In testing with similar pressure they found that moisture in compressed air formed after few seconds of decompressing and that removing moisture from compressed air could avoid this problem.
Space shuttle engines can generate enough heat to vaporize it but they cool the engines by using liquid hydrogen as fuel and it cool down as depressurizing hydrogen cools it near 33 kelvins. Hydrogen tubes reach around engine and go inside were they spread through over thousand parallel metal pipes that don't melt due to cooling.
Monday, July 23, 2012
Basics of optics
In short materials and environments (air, vacuum, water, glass, diamonds) that don't conduct electricity are transparent if they don't have cracks or scrapes. Materials that conduct electricity (metals, graphite and pure silicon) are nontransparent and they can reflect light. For example thin metal foil can reflect light even if that traveled several light-years through space and atmosphere. In addition light reflects partially between areas that have different electric conductivity like between air and water or air and random nonconducting solid.
Cracks can make otherwise transparent materials like glass nontransparent by making it move semi-randomly in many directions. If light enters material with different refraction index and different local speed of light then it bends somewhat and cracks
For transparency material shouldn't have cracks and scrapes larger than the wavelength of visible light (around 0,5 micrometers).
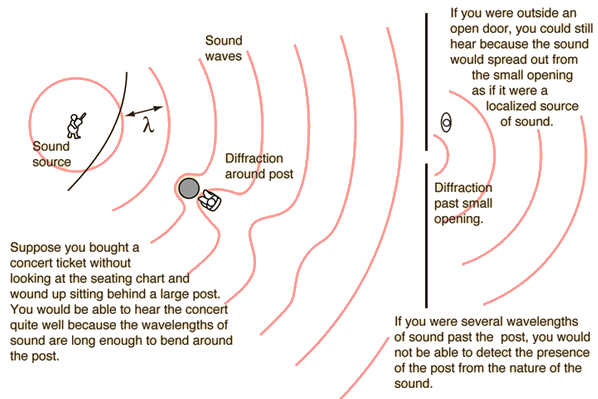
Light sound waves visible light waves also bend around things that are shorter than wavelength so within couple of wavelength waves straighten out and continue behind small objects. That's also the reason why radio waves used in radars have to be smaller than object to detect it and x-rays with wavelength similar to molecules can make molecular structure visible.
Cracks can make otherwise transparent materials like glass nontransparent by making it move semi-randomly in many directions. If light enters material with different refraction index and different local speed of light then it bends somewhat and cracks
For transparency material shouldn't have cracks and scrapes larger than the wavelength of visible light (around 0,5 micrometers).

Light sound waves visible light waves also bend around things that are shorter than wavelength so within couple of wavelength waves straighten out and continue behind small objects. That's also the reason why radio waves used in radars have to be smaller than object to detect it and x-rays with wavelength similar to molecules can make molecular structure visible.
Possibly useful HIV treatment by modifing bone marrow
HIV needs 2 specific receptors on T-cells to enter them and multiply. One is CD4 and other 2 are CCR5 and CXCR4. Those last 2 are receptors to chemokines that regulate movements of white blood cells. If those 2 are mutated enough then HIV can't enter them and it seems people with these mutations don't get infected with HIV without having serious health problems.
For example if all human ccr5 receptor genes have ccr5Δ32 mutation (32 base pairs missing) then they don't produce CCR5 and their T-cells don't get infected with R-5 (CCR5-tropic) HIV strain and those who have one mutated copy in one of the chromosome produce less CCR5 and tend to live longer after HIV infections. If HIV type uses CXCR4 then this previous mutation would not protect against infection.
Authors of this study decided to use proteins (zinc-finger nuclease) that break DNA from certain places so cxcr4 genes would mutate due to errors that happen when DNA parts rejoin. Using these proteins seemed to protect T-cells from HIV strains (X-4 tropic) that used CXCR4 for entering T-cells. If CCR5 and CXCR4 both got mutated enough then the protective effects improved.
Cells grown outside body seemed to tolerate these added mutations and kept multiplying.
Above illustration shows how much HIV affected different T-cells and how many were alive in different days. NTD is normal T-cell. R5ZFN is zinc finger nuclease that damages CCR5 and X4ZFN mutates CXCR4. X4ZFN seemed most protective against the 3 HIV strains they tested on T-cells without much cell death after HIV infection.
In one case a HIV positive patient got bone marrow due to leukemia and donor had ccr5Δ32 gene versions. Authors also noted that they know about humans with large CXCR4 mutations and that CXCR4 is involved in development of brain and cardiovascular system so mutation of that gene are probably deadly for fetus although mutations with ZFN in adults seem tolerable. As blood cells are produced in marrow it also caused production of mutated T-cells. After transplantation his HIV viral load remained undetectable even 3 years after transplantation.
Authors said it could be first "HIV cure" although transplantation of tissue from other person means patient has to take immune weakening drugs that may cause more serious infections and tumors. Also such mutations are rare for transplantations to all HIV positive patients. One alternative they offered was to use ZFN proteins to produce marrow with patients own cells that would produce HIV resistant T-cells.
My comment: if CXCR4 are needed to grow cardiovascular system and brain then mutations in them could make having children impossible if patients CXCR4 genes would get mutated in all their cells but authors wrote mostly about removing tissue, mutating it and re-transplanting.
Sunday, July 1, 2012
Strengthening materials
Materials such as ceramics, glasses and metals could be strengthened by adding extra chemical element that could form connections with as many atoms as possible. In case of chemical bonds that's with around 4 atoms and such elements are in middle of periodic table away from left and right edge.
Electrostatic attraction is other force that hold many materials together. Even hydrogen bond in water is example of electrostatic attraction and it helps with increasing melting and boiling temperatures.
Electronegativity shows how strongly some element attracts electrons. Substances with higher electronegativity usually get negative charge and the ones with smaller values get more positive charge in at least ionic bonds. That's partly because less electronegative elements have larger atoms and their electron are further away from positive charge in nucleus making these electrons easy to remove (giving atom positive charge) while in top right corner of periodic table atoms are smallest and positive charge of nucleus is closer to all its electrons so the strong attraction can give it extra electron(s).
Soft metals have very weak structure. For example sodium, lithium and other group 1 elements only have 1 electron for chemical bond so they could only form strong connection with 1 other atom and that makes these metals very soft and fragile. This lack of connection with other atoms may explain why these metals have low melting temperature (lithium melts at 180 C and sodium at 97 C).
One early example of material strengthening was in production of steel. Iron is fragile with too low or too high carbon % but if carbon forms 0,2-2,1% of steel by weight then this mixture can get extra strength. Usually at first carbon was burned out by pumping oxygen into melted iron mix to burn it away as CO2. After that people add required amount of carbon into molten metal in atmosphere that can't burn carbon like argon, helium and other noble gases.
These solutions may be common in production of other materials because often different elements get mixed in liquid hot environment where different ingredients melt and evaporate at different temperatures.
Carbon has more negative charge (more electronegative) than iron and that somewhat large difference in electronegativity can give steel strong electrostatic pull. Although oxygen and fluorine have higher electronegativity they have much less free electrons for forming bonds.
Iron has cubic body centered crystal structure that has 9 iron atoms in crystal unit. In case of 2% carbon it would mean about 10% of atoms are carbon and that makes about 1 carbon atom for each such cubical unit in iron crystal although iron could also have structure shown in middle.
In case of clay and porcelain diversity of ingredients can also help with durability.
Bone china is one more historic type of porcelain that Europeans learned to produce. One recipe for it is to mix kaolinite (25%), feldspar (25%) and bone ash (50%). Kaolinite (Al2S2O5(OH)4) is basically clean clay. Feldspar has also aluminium, silicon, oxygen and hydrogen but in addition sodium, calcium and potassium. Bone ash is left after burning bones and that composes mostly of phosphate groups and calcium (CaHPO4).
Special thing about bone ash is that both phosphate and calcium have double charges. Calcium has +2 charge and phosphate group has -2 charge making electrostatic with them extra strong and porcelain was partly valued because it resisted drops to floor better than clay.
Elements found in clay can also be used to produce very strong and scratch resistant transparent glass (porcelain is also somewhat transparent). One version of this strong glass has brand name Gorilla Glass and it used on newer more expensive phones and tablets. It is made from aluminum, silicon and oxygen like clay with additional potassium and sodium. After glass gets made from Al, Si, O and Na it gets strengthened by putting it in molten potassium salt at 400 C. Smaller sodium ions leave glass and larger potassium moves into glass that expanded during heating. After cooling glass constricts again and forces atoms tighter together but now atom are more densely packed due to larger potassium with larger differences in electronegativity between elements (and stronger electrostatic attraction) than before.
Example of Gorilla Glass in use.
Subscribe to:
Comments (Atom)